Synthesis of Benzimidazole-Sulfonyl Derivatives and Their Biological Activities
- PMID: 35425644
- PMCID: PMC9005321
- DOI: 10.1155/2022/7255299
Synthesis of Benzimidazole-Sulfonyl Derivatives and Their Biological Activities
Abstract
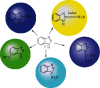
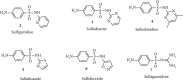
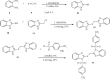
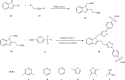
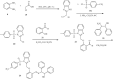
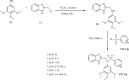
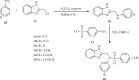
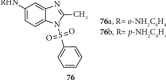
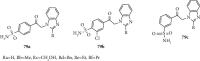
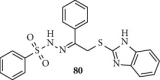
Currently, the synthesis of new compounds with potential bioactivities has become a central issue in the drug discovery arena. Among these new compounds, benzimidazole-sulfonyl scaffolds have vital applications in the fields of pharmaceuticals industries. Benzimidazole and sulfonyl compounds have remarkable biological activities, such as antibacterial, antifungal, anti-inflammatory, antiproliferative, carbonic anhydrase inhibitory, and α-amylase inhibitory activities. Furthermore, recent literature mentions the synthesis and bioactivities of some benzimidazole-sulfonyl hybrids. In this review, we focus on reviewing the synthesis of these hybrid scaffolds and their various types of biological activities of the compounds.
Copyright © 2022 Endale Mulugeta and Yoseph Samuel.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
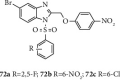

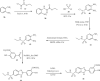
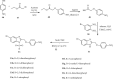
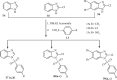
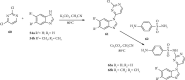
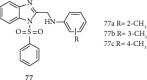

References
-
- Thakral S., Singh V. Recent development on importance of heterocyclic amides as potential bioactive molecules: a review. Current Bioactive Compounds . 2019;15(3):316–336. doi: 10.2174/1573407214666180614121140. - DOI
Publication types
LinkOut - more resources
Full Text Sources

