A prospective cohort study to evaluate the incidence of febrile neutropenia in patients receiving pegfilgrastim on-body injector versus other options for prophylaxis of febrile neutropenia: breast cancer subgroup analysis
- PMID: 35426046
- PMCID: PMC9009498
- DOI: 10.1007/s00520-022-07025-2
A prospective cohort study to evaluate the incidence of febrile neutropenia in patients receiving pegfilgrastim on-body injector versus other options for prophylaxis of febrile neutropenia: breast cancer subgroup analysis
Abstract
Background: Breast cancer chemotherapy often carries a high risk of febrile neutropenia (FN); guidelines recommend prophylaxis with granulocyte colony-stimulating factor (G-CSF), such as pegfilgrastim. Neulasta® Onpro® on-body injector (OBI) is a delivery device administering pegfilgrastim approximately 27 h after application.
Methods: This prospective study examined patients with breast cancer who received chemotherapy with a high risk of FN, receiving OBI ("OBI") or other options (other G-CSF or none; "other"). The primary endpoint was FN incidence; secondary endpoints included chemotherapy delivery, adherence (G-CSF in all cycles), compliance (G-CSF day after chemotherapy), and FN incidence in patients receiving curative or palliative treatment.
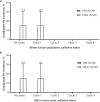
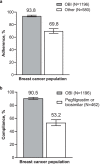
Results: A total of 1776 patients with breast cancer were enrolled (OBI, n = 1196; other, n = 580). Across all cycles, FN incidence was lower for OBI (4.4% [95% CI, 3.3-5.6%]) than other (7.4% [5.3-9.6%]). For curative treatment, the FN incidence across all cycles was lower for OBI (4.6% [3.4-5.8%]) than for other (7.1% [5.0-9.3%]). For palliative treatment (OBI, n = 33; other, n = 20), 3 patients (15%) in the other and none in the OBI group had FN. After adjusting for baseline covariates, FN incidence remained lower for OBI (4.6% [3.5-6.1%]) versus other (7.8% [5.7-10.5%]). Adherence was higher for OBI (93.8%) than for other G-CSF (69.8%), as was compliance (90.5 and 53.2%, respectively). Chemotherapy dose delays/reductions were similar for OBI (4.7%/32.3%, respectively) and other (4.7%/30.0%) groups.
Conclusion: Pegfilgrastim OBI was associated with a lower FN incidence in patients with breast cancer compared to other options for FN prophylaxis.
Trial registration: www.
Clinicaltrials: gov , NCT02178475, registered 30 June, 2014.
Keywords: Breast cancer; Chemotherapy; Compliance; Febrile neutropenia; Pegfilgrastim.
© 2022. The Author(s).
Conflict of interest statement
Reshma L. Mahtani reports consulting fees from Biotheranostics, Amgen, Agendia, Eisai, Immunomedics, Genentech, Lilly, Novartis, Merck, Pfizer, Puma, Sanofi, Seagen, AstraZeneca, Daiichi, and participation on an advisory board for AstraZeneca, Amgen, Agendia, Biotheranostics, Eisai, Immunomedics, Lilly, Novartis, Merck, Pfizer, Puma, Sanofi, Seagen, and Daiichi; Rajesh Belani reports employment with Poseida Therapeutics and stock/stock options in Amgen; Jeffrey Crawford reports consulting fees from GlaxoSmithKline, G1 Therapeutics, Merck, Pfizer, Spectrum, and participation on a data safety monitoring board or advisory board for Beyond Spring, G1 Therapeutics, Merrimack, Mylan and Roche; David Dale reports consulting fees from Amgen, and contracted research support from Amgen; Lucy DeCosta reports employment with Amgen Inc.; Prasad L. Gawade reports employment and stockholding with Amgen Inc.; Chanh Huynh has no disclosures to report; Tatiana Lawrence reports employment and stockholding with Amgen Inc.; Sandra Lewis reports employment and stockholding with Amgen Inc.; William W. MacLaughlin and Mohit Narang have no disclosures to report; Robert Rifkin reports participation in advisory boards for Amgen, BMS (Celgene), Coherus, Fresenius-Kabi, GSK, Janssen, and Pfizer.
Figures


References
-
- Pathak R, Giri S, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Mortality, length of stay, and health care costs of febrile neutropenia-related hospitalizations among patients with breast cancer in the United States. Support Care Cancer. 2015;23:615–617. doi: 10.1007/s00520-014-2553-0. - DOI - PubMed
-
- Averin A, Silvia A, Lamerato L, Richert-Boe K, Kaur M, Sundaresan D, Shah N, Hatfield M, Lawrence T, Lyman GH, Weycker D. Risk of chemotherapy-induced febrile neutropenia in patients with metastatic cancer not receiving granulocyte colony-stimulating factor prophylaxis in US clinical practice. Support Care Cancer. 2021;29:2179–2186. doi: 10.1007/s00520-020-05715-3. - DOI - PMC - PubMed
-
- Lyman GH, Dale DC, Culakova E, Poniewierski MS, Wolff DA, Kuderer NM, Huang M, Crawford J. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24:2475–2484. doi: 10.1093/annonc/mdt226. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

