Targeting mutations in cancer
- PMID: 35426374
- PMCID: PMC9012285
- DOI: 10.1172/JCI154943
Targeting mutations in cancer
Abstract
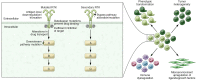
Targeted therapies have come to play an increasingly important role in cancer therapy over the past two decades. This success has been made possible in large part by technological advances in sequencing, which have greatly advanced our understanding of the mutational landscape of human cancer and the genetic drivers present in individual tumors. We are rapidly discovering a growing number of mutations that occur in targetable pathways, and thus tumor genetic testing has become an important component in the choice of appropriate therapies. Targeted therapy has dramatically transformed treatment outcomes and disease prognosis in some settings, whereas in other oncologic contexts, targeted approaches have yet to demonstrate considerable clinical efficacy. In this Review, we summarize the current knowledge of targetable mutations that occur in a range of cancers, including hematologic malignancies and solid tumors such as non-small cell lung cancer and breast cancer. We outline seminal examples of druggable mutations and targeting modalities and address the clinical and research challenges that must be overcome to maximize therapeutic benefit.
Conflict of interest statement
Figures


References
-
- Cheng DT, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

