Anti-Parkinsonian Therapy: Strategies for Crossing the Blood-Brain Barrier and Nano-Biological Effects of Nanomaterials
- PMID: 35426525
- PMCID: PMC9012800
- DOI: 10.1007/s40820-022-00847-z
Anti-Parkinsonian Therapy: Strategies for Crossing the Blood-Brain Barrier and Nano-Biological Effects of Nanomaterials
Abstract
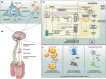
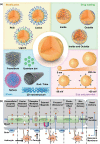
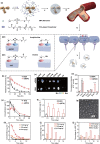
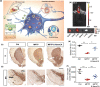
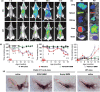
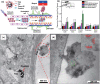
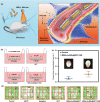
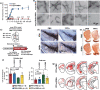
Parkinson's disease (PD), a neurodegenerative disease that shows a high incidence in older individuals, is becoming increasingly prevalent. Unfortunately, there is no clinical cure for PD, and novel anti-PD drugs are therefore urgently required. However, the selective permeability of the blood-brain barrier (BBB) poses a huge challenge in the development of such drugs. Fortunately, through strategies based on the physiological characteristics of the BBB and other modifications, including enhancement of BBB permeability, nanotechnology can offer a solution to this problem and facilitate drug delivery across the BBB. Although nanomaterials are often used as carriers for PD treatment, their biological activity is ignored. Several studies in recent years have shown that nanomaterials can improve PD symptoms via their own nano-bio effects. In this review, we first summarize the physiological features of the BBB and then discuss the design of appropriate brain-targeted delivery nanoplatforms for PD treatment. Subsequently, we highlight the emerging strategies for crossing the BBB and the development of novel nanomaterials with anti-PD nano-biological effects. Finally, we discuss the current challenges in nanomaterial-based PD treatment and the future trends in this field. Our review emphasizes the clinical value of nanotechnology in PD treatment based on recent patents and could guide researchers working in this area in the future.
Keywords: Biomimetic drug delivery; Blood–brain barrier; Nano-biological effects; Nasal delivery; Parkinson’s disease.
© 2022. The Author(s).
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
