Vaccine protection against the SARS-CoV-2 Omicron variant in macaques
- PMID: 35427477
- PMCID: PMC8926910
- DOI: 10.1016/j.cell.2022.03.024
Vaccine protection against the SARS-CoV-2 Omicron variant in macaques
Abstract
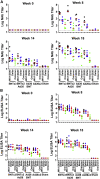
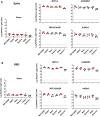
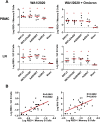
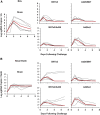
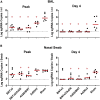
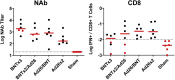
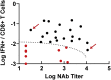
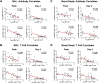
The rapid spread of the SARS-CoV-2 Omicron (B.1.1.529) variant, including in highly vaccinated populations, has raised important questions about the efficacy of current vaccines. In this study, we show that the mRNA-based BNT162b2 vaccine and the adenovirus-vector-based Ad26.COV2.S vaccine provide robust protection against high-dose challenge with the SARS-CoV-2 Omicron variant in cynomolgus macaques. We vaccinated 30 macaques with homologous and heterologous prime-boost regimens with BNT162b2 and Ad26.COV2.S. Following Omicron challenge, vaccinated macaques demonstrated rapid control of virus in bronchoalveolar lavage, and most vaccinated animals also controlled virus in nasal swabs. However, 4 vaccinated animals that had moderate Omicron-neutralizing antibody titers and undetectable Omicron CD8+ T cell responses failed to control virus in the upper respiratory tract. Moreover, virologic control correlated with both antibody and T cell responses. These data suggest that both humoral and cellular immune responses contribute to vaccine protection against a highly mutated SARS-CoV-2 variant.
Keywords: Omicron; SARS-CoV-2; macaque.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.H.B. is a co-inventor on provisional vaccine patents licensed to Janssen (63/121,482; 63/133,969; 63/135,182) and serves as a consultant to Pfizer. The authors report no other conflict of interest. A.C.M.B. has received funding from Abbvie for the commercial development of SARS-CoV-2 mAbs.
Figures





Update of
-
Vaccine Protection Against the SARS-CoV-2 Omicron Variant in Macaques.bioRxiv [Preprint]. 2022 Feb 7:2022.02.06.479285. doi: 10.1101/2022.02.06.479285. bioRxiv. 2022. Update in: Cell. 2022 Apr 28;185(9):1549-1555.e11. doi: 10.1016/j.cell.2022.03.024. PMID: 35169798 Free PMC article. Updated. Preprint.
References
-
- Carreño J.M., Alshammary H., Tcheou J., Singh G., Raskin A.J., Kawabata H., Sominsky L.A., Clark J.J., Adelsberg D.C., Bielak D.A., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–688. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

