Public health impact and cost-effectiveness of gonorrhoea vaccination: an integrated transmission-dynamic health-economic modelling analysis
- PMID: 35427491
- PMCID: PMC9217755
- DOI: 10.1016/S1473-3099(21)00744-1
Public health impact and cost-effectiveness of gonorrhoea vaccination: an integrated transmission-dynamic health-economic modelling analysis
Erratum in
-
Correction to Lancet Infect Dis 2022; published online April 12. https://doi.org/10.1016/S1473-3099(21)00744-1.Lancet Infect Dis. 2022 Jun;22(6):e159. doi: 10.1016/S1473-3099(22)00280-8. Epub 2022 Apr 29. Lancet Infect Dis. 2022. PMID: 35500591 Free PMC article. No abstract available.
Abstract
Background: Gonorrhoea is a rapidly growing public health threat, with rising incidence and increasing drug resistance. Evidence that the MeNZB and four-component serogroup B meningococcal (4CMenB) vaccines, designed against Neisseria meningitidis, can also offer protection against gonorrhoea has created interest in using 4CMenB for this purpose and for developing gonorrhoea-specific vaccines. However, cost-effectiveness, and how the efficacy and duration of protection affect a gonorrhoea vaccine's value, have not been assessed.
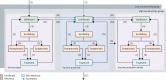
Methods: We developed an integrated transmission-dynamic health-economic model, calibrated using Bayesian methods to surveillance data (from the Genitourinary Medicine Clinic Activity Dataset and the Gonococcal Resistance to Antimicrobials Surveillance Programme) on men who have sex with men (MSM) in England. We considered vaccination of MSM from the perspective of sexual health clinics, with and without vaccination offered to all adolescents in schools (vaccination before entry [VbE]), comparing three realistic approaches to targeting: vaccination on attendance (VoA) for testing; vaccination on diagnosis (VoD) with gonorrhoea; or vaccination according to risk (VaR), offered to patients diagnosed with gonorrhoea plus individuals who test negative but report having more than five sexual partners per year. For the primary analysis, vaccine impact was assessed relative to no vaccination in a conservative baseline scenario wherein time-varying behavioural parameters (sexual risk behaviour and screening rates) stabilise. To calculate the value of vaccination per dose administered, the value of vaccination was calculated by summing the averted costs of testing and treatment, and the monetary value of quality-adjusted life-year (QALY) gains with a QALY valued at £20 000. Costs were in 2018-19 GB£, and both costs and QALYs were discounted at 3·5% per year. We analysed the effects of varying vaccine uptake (0·5, 1, or 2 times HPV vaccine uptake by MSM in sexual health clinics in England), vaccine efficacy (1-100%) and duration of protection (1-20 years), and the time-horizon considered (10 years and 20 years). In addition, we calculated incremental cost-effectiveness ratios for the use of 4CMenB using assumed vaccine prices.
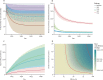
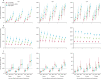
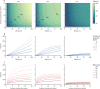
Findings: VbE has little impact on gonorrhoea diagnoses, with only 1·7% of MSM vaccinated per year. VoA has the largest impact but requires more vaccine doses than any other strategy, whereas VoD has a moderate impact but requires many fewer doses than VoA. VaR has almost the same impact as VoA but with fewer doses administered than VoA. VaR is the most cost-effective strategy for vaccines of moderate efficacy or duration of protection (or both), although VoD is more cost-effective for very protective and long-lasting vaccines. Even under conservative assumptions (efficacy equivalent to that of MeNZB and protection lasting for 18 months after two-dose primary vaccination and 36 months after single-dose booster vaccination), 4CMenB administered under VaR would likely be cost-saving at its current National Health Service price, averting an estimated mean 110 200 cases (95% credible interval 36 500-223 600), gaining a mean 100·3 QALYs (31·0-215·8), and saving a mean £7·9 million (0·0-20·5) over 10 years. A hypothetical gonorrhoea vaccine's value is increased more by improving its efficacy than its duration of protection-eg, 30% protection lasting 2 years has a median value of £48 (22-85) per dose over 10 years; doubling efficacy increases the value to £102 (53-144) whereas doubling the duration of protection increases it to £72 (34-120).
Interpretation: We recommend that vaccination of MSM against gonorrhoea according to risk in sexual health clinics in England with the 4CMenB vaccine be considered. Development of gonorrhoea-specific vaccines should prioritise maximising efficacy over duration of protection.
Funding: Medical Research Council (UK), National Institute for Health Research (UK).
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests PJW has received payment from Pfizer for teaching of mathematical modelling of infectious disease transmission and vaccination. All other authors declare no competing interests.
Figures
Comment in
-
Feasibility of gonorrhoea vaccination among men who have sex with men in England.Lancet Infect Dis. 2022 Jul;22(7):921-923. doi: 10.1016/S1473-3099(21)00760-X. Epub 2022 Apr 12. Lancet Infect Dis. 2022. PMID: 35427489 No abstract available.
References
-
- Public Health England Sexually transmitted infections (STIs): annual data tables. June 17, 2010. https://www.gov.uk/government/statistics/sexually-transmitted-infections...
-
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17:e235–e279. - PubMed
-
- Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390:1603–1610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

