Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder
- PMID: 35427534
- PMCID: PMC11197974
- DOI: 10.1016/S2213-2600(21)00510-5
Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder
Abstract
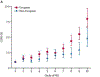
Chronic obstructive pulmonary disease (COPD) is a deadly and highly morbid disease. Susceptibility to and heterogeneity of COPD are incompletely explained by environmental factors such as cigarette smoking. Family-based and population-based studies have shown that a substantial proportion of COPD risk is related to genetic variation. Genetic association studies have identified hundreds of genetic variants that affect risk for COPD, decreased lung function, and other COPD-related traits. These genetic variants are associated with other pulmonary and non-pulmonary traits, demonstrate a genetic basis for at least part of COPD heterogeneity, have a substantial effect on COPD risk in aggregate, implicate early-life events in COPD pathogenesis, and often involve genes not previously suspected to have a role in COPD. Additional progress will require larger genetic studies with more ancestral diversity, improved profiling of rare variants, and better statistical methods. Through integration of genetic data with other omics data and comprehensive COPD phenotypes, as well as functional description of causal mechanisms for genetic risk variants, COPD genetics will continue to inform novel approaches to understanding the pathobiology of COPD and developing new strategies for management and treatment.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MHC and EKS have received grant support from GlaxoSmithKline and Bayer. MHC has received consulting and speaking fees from Illumina and AstraZeneca. BDH declares no competing interests.
Figures
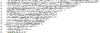

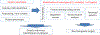
Comment in
-
GETting to know the many causes and faces of COPD.Lancet Respir Med. 2022 May;10(5):426-428. doi: 10.1016/S2213-2600(22)00049-2. Epub 2022 Apr 12. Lancet Respir Med. 2022. PMID: 35427529 No abstract available.
-
COPD, smoking, and social justice.Lancet Respir Med. 2022 May;10(5):428-430. doi: 10.1016/S2213-2600(22)00130-8. Epub 2022 Apr 12. Lancet Respir Med. 2022. PMID: 35427531 No abstract available.
References
-
- Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis [Internet]. Am Rev Respir Dis; 1977. Feb [cited 2021 Feb 22];115(2):195–205. Available from: http://www.ncbi.nlm.nih.gov/pubmed/842934 - PubMed
-
- Marsh S, Aldington S, Shirtcliffe P, Weatherall M, Beasley R. Smoking and COPD: What really are the risks? Eur Respir J [Internet]. Eur Respir J; 2006. Oct [cited 2021 Feb 22];28(4):883–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17012635 - PubMed
-
- Zhou JJ, Cho MH, Castaldi PJ, Hersh CP, Silverman EK, Laird NM. Heritability of Chronic Obstructive Pulmonary Disease and Related Phenotypes in Smokers. Am J Respir Crit Care Med [Internet]. 2013. Aug 23 [cited 2013 Aug 27];188(8):941–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23972146 - PMC - PubMed
-
- Silverman EK, Chapman H a, Drazen JM, Weiss ST, Rosner B, Campbell EJ, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med [Internet]. 1998. Jun;157(6 Pt 1):1770–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9620904 - PubMed
-
- Polderman TJC, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet [Internet]. 2015. Jul 18 [cited 2019 Jul 25];47(7):702–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25985137 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

