Multi-label annotation of text reports from computed tomography of the chest, abdomen, and pelvis using deep learning
- PMID: 35428335
- PMCID: PMC9011942
- DOI: 10.1186/s12911-022-01843-4
Multi-label annotation of text reports from computed tomography of the chest, abdomen, and pelvis using deep learning
Abstract
Background: There is progress to be made in building artificially intelligent systems to detect abnormalities that are not only accurate but can handle the true breadth of findings that radiologists encounter in body (chest, abdomen, and pelvis) computed tomography (CT). Currently, the major bottleneck for developing multi-disease classifiers is a lack of manually annotated data. The purpose of this work was to develop high throughput multi-label annotators for body CT reports that can be applied across a variety of abnormalities, organs, and disease states thereby mitigating the need for human annotation.
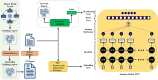
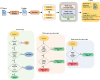
Methods: We used a dictionary approach to develop rule-based algorithms (RBA) for extraction of disease labels from radiology text reports. We targeted three organ systems (lungs/pleura, liver/gallbladder, kidneys/ureters) with four diseases per system based on their prevalence in our dataset. To expand the algorithms beyond pre-defined keywords, attention-guided recurrent neural networks (RNN) were trained using the RBA-extracted labels to classify reports as being positive for one or more diseases or normal for each organ system. Alternative effects on disease classification performance were evaluated using random initialization or pre-trained embedding as well as different sizes of training datasets. The RBA was tested on a subset of 2158 manually labeled reports and performance was reported as accuracy and F-score. The RNN was tested against a test set of 48,758 reports labeled by RBA and performance was reported as area under the receiver operating characteristic curve (AUC), with 95% CIs calculated using the DeLong method.
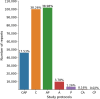
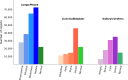
Results: Manual validation of the RBA confirmed 91-99% accuracy across the 15 different labels. Our models extracted disease labels from 261,229 radiology reports of 112,501 unique subjects. Pre-trained models outperformed random initialization across all diseases. As the training dataset size was reduced, performance was robust except for a few diseases with a relatively small number of cases. Pre-trained classification AUCs reached > 0.95 for all four disease outcomes and normality across all three organ systems.
Conclusions: Our label-extracting pipeline was able to encompass a variety of cases and diseases in body CT reports by generalizing beyond strict rules with exceptional accuracy. The method described can be easily adapted to enable automated labeling of hospital-scale medical data sets for training image-based disease classifiers.
Keywords: Attention RNN; Computed tomography; Natural language processing; Report labeling; Rule-based algorithm; Weak supervision.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Solti I, Cooke CR, Xia F, Wurfel MM, editors. Automated classification of radiology reports for acute lung injury: comparison of keyword and machine learning based natural language processing approaches. In 2009 IEEE international conference on bioinformatics and biomedicine workshop; 2009: IEEE. - PMC - PubMed
-
- Wang X, Peng Y, Lu L, Lu Z, Bagheri M, Summers RM. ChestX-Ray8: hospital-scale chest X-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In: 2017 IEEE conference on computer vision and pattern recognition (CVPR), 2017, pp. 3462–71.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

