Which actionable statements qualify as good practice statements In Covid-19 guidelines? A systematic appraisal
- PMID: 35428695
- PMCID: PMC9044517
- DOI: 10.1136/bmjebm-2021-111866
Which actionable statements qualify as good practice statements In Covid-19 guidelines? A systematic appraisal
Abstract
Objectives: To evaluate the development and quality of actionable statements that qualify as good practice statements (GPS) reported in COVID-19 guidelines.
Design and setting: Systematic review . We searched MEDLINE, MedSci, China National Knowledge Infrastructure (CNKI), databases of Grading of Recommendations Assessment, Development and Evaluation (GRADE) Guidelines, NICE, WHO and Guidelines International Network (GIN) from March 2020 to September 2021. We included original or adapted recommendations addressing any COVID-19 topic.
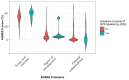
Main outcome measures: We used GRADE Working Group criteria for assessing the appropriateness of issuing a GPS: (1) clear and actionable; (2) rationale necessitating the message for healthcare practice; (3) practicality of systematically searching for evidence; (4) likely net positive consequences from implementing the GPS and (5) clear link to the indirect evidence. We assessed guideline quality using the Appraisal of Guidelines for Research and Evaluation II tool.
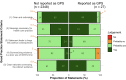
Results: 253 guidelines from 44 professional societies issued 3726 actionable statements. We classified 2375 (64%) as GPS; of which 27 (1%) were labelled as GPS by guideline developers. 5 (19%) were labelled as GPS by their authors but did not meet GPS criteria. Of the 2375 GPS, 85% were clear and actionable; 59% provided a rationale necessitating the message for healthcare practice, 24% reported the net positive consequences from implementing the GPS. Systematic collection of evidence was deemed impractical for 13% of the GPS, and 39% explained the chain of indirect evidence supporting GPS development. 173/2375 (7.3%) statements explicitly satisfied all five criteria. The guidelines' overall quality was poor regardless of the appropriateness of GPS development and labelling.
Conclusions: Statements that qualify as GPS are common in COVID-19 guidelines but are characterised by unclear designation and development processes, and methodological weaknesses.
Keywords: COVID-19; Evidence-Based Practice; Health Services Research.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HJS, AS, VAW report grants from Canadian Instituites of Health during the conduct of the study—FRN VR4-172741 & GA3-177732. RAM reports grants from WHO, grants from ASH, grants from ACR, other from Boehringer ingelheim international, grants from NIDDK, outside the submitted work. EA, PA-C and HJS report contribution to the development of the original five criteria for assessing the appropriateness of issuing good practice statements. The remaining authors have nothing else to declare.
Figures