Signal voids of active cardiac implants at 3.0 T CMR
- PMID: 35428775
- PMCID: PMC9014817
- DOI: 10.1038/s41598-022-09690-z
Signal voids of active cardiac implants at 3.0 T CMR
Abstract
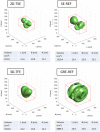
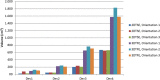
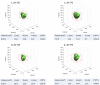
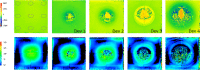
Recent technical advancements allow cardiac MRI (CMR) examinations in the presence of so-called MRI conditional active cardiac implants at 3.0 T. However, the artifact burden caused by susceptibility effects remain an obstacle. All measurements were obtained at a clinical 3.0 T scanner using an in-house designed cubic phantom and optimized sequences for artifact evaluation (3D gradient echo sequence, multi-slice 2D turbo spin echo sequence). Reference sequences according to the American Society for Testing and Materials (ASTM) were additionally applied. Four representative active cardiac devices and a generic setup were analyzed regarding volume and shape of the signal void. For analysis, a threshold operation was applied to the grey value profile of each data set. The presented approach allows the evaluation of the signal void and shape even for larger implants such as ICDs. The void shape is influenced by the orientation of the B0-field and by the chosen sequence type. The distribution of ferromagnetic material within the implants also matters. The void volume depends both on the device itself, and on the sequence type. Disturbances in the B0 and B1 fields exceed the visual signal void. This work presents a reproducible and highly defined approach to characterize both signal void artifacts at 3.0 T and their influencing factors.
© 2022. The Author(s).
Conflict of interest statement
Dr. Weiss is an employee of BIOTRONIK SE & Co. KG, Berlin, Germany, and Dr. Weber is an employee of Philips GmbH, Hamburg, Germany. Prof. Bauer is a scientific advisor for BIOTRONIK SE & Co. KG, Berlin, Germany. Also, this work has been partially funded by BIOTRONIK SE & Co. KG, Berlin, Germany. Dr. Reiter has no competing interests to declare.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

