Propagation of seminal toxins through binary expression gene drives could suppress populations
- PMID: 35428855
- PMCID: PMC9012762
- DOI: 10.1038/s41598-022-10327-4
Propagation of seminal toxins through binary expression gene drives could suppress populations
Abstract
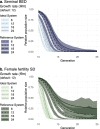
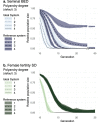
Gene drives can be highly effective in controlling a target population by disrupting a female fertility gene. To spread across a population, these drives require that disrupted alleles be largely recessive so as not to impose too high of a fitness penalty. We argue that this restriction may be relaxed by using a double gene drive design to spread a split binary expression system. One drive carries a dominant lethal/toxic effector alone and the other a transactivator factor, without which the effector will not act. Only after the drives reach sufficiently high frequencies would individuals have the chance to inherit both system components and the effector be expressed. We explore through mathematical modeling the potential of this design to spread dominant lethal/toxic alleles and suppress populations. We show that this system could be implemented to spread engineered seminal proteins designed to kill females, making it highly effective against polyandrous populations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Dara SK. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manage. 2019;10:25. doi: 10.1093/jipm/pmz010. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

