Viral and cellular translation during SARS-CoV-2 infection
- PMID: 35429230
- PMCID: PMC9110871
- DOI: 10.1002/2211-5463.13413
Viral and cellular translation during SARS-CoV-2 infection
Abstract
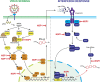
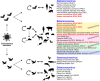
SARS-CoV-2 is a betacoronavirus that emerged in China in December 2019 and which is the causative agent of the Covid-19 pandemic. This enveloped virus contains a large positive-sense single-stranded RNA genome. In this review, we summarize the current knowledge on the molecular mechanisms for the translation of both viral transcripts and cellular messenger RNAs. Non-structural proteins are encoded by the genomic RNA and are produced in the early steps of infection. In contrast, the structural proteins are produced from subgenomic RNAs that are translated in the late phase of the infectious program. Non-structural protein 1 (NSP1) is a key molecule that regulates both viral and cellular translation. In addition, NSP1 interferes with multiple steps of the interferon I pathway and thereby blocks host antiviral responses. Therefore, NSP1 is a drug target of choice for the development of antiviral therapies.
Keywords: NSP1-SL1; SARS-CoV-2; immune response; interferon; ribosome; translation.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



Similar articles
-
Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation.Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2017715118. doi: 10.1073/pnas.2017715118. Proc Natl Acad Sci U S A. 2021. PMID: 33479166 Free PMC article.
-
The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS-CoV-2 translation.RNA. 2020 Dec 2;27(3):253-64. doi: 10.1261/rna.078121.120. Online ahead of print. RNA. 2020. PMID: 33268501 Free PMC article.
-
Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2.Science. 2020 Sep 4;369(6508):1249-1255. doi: 10.1126/science.abc8665. Epub 2020 Jul 17. Science. 2020. PMID: 32680882 Free PMC article.
-
I(nsp1)ecting SARS-CoV-2-ribosome interactions.Commun Biol. 2021 Jun 10;4(1):715. doi: 10.1038/s42003-021-02265-0. Commun Biol. 2021. PMID: 34112887 Free PMC article. Review.
-
Mechanisms of Coronavirus Nsp1-Mediated Control of Host and Viral Gene Expression.Cells. 2021 Feb 2;10(2):300. doi: 10.3390/cells10020300. Cells. 2021. PMID: 33540583 Free PMC article. Review.
Cited by
-
Curcumin partly prevents ISG15 activation via ubiquitin-activating enzyme E1-like protein and decreases ISGylation.Biochem Biophys Res Commun. 2022 Oct 15;625:94-101. doi: 10.1016/j.bbrc.2022.08.003. Epub 2022 Aug 5. Biochem Biophys Res Commun. 2022. PMID: 35952613 Free PMC article.
-
Inhibition of Viral RNA-Dependent RNA Polymerases by Nucleoside Inhibitors: An Illustration of the Unity and Diversity of Mechanisms.Int J Mol Sci. 2022 Oct 21;23(20):12649. doi: 10.3390/ijms232012649. Int J Mol Sci. 2022. PMID: 36293509 Free PMC article. Review.
-
Innovation and renovation: FEBS Open Bio in 2023.FEBS Open Bio. 2023 Jan;13(1):4-9. doi: 10.1002/2211-5463.13531. FEBS Open Bio. 2023. PMID: 36594366 Free PMC article.
-
Recognition and cleavage of human tRNA methyltransferase TRMT1 by the SARS-CoV-2 main protease.Elife. 2025 Jan 7;12:RP91168. doi: 10.7554/eLife.91168. Elife. 2025. PMID: 39773525 Free PMC article.
-
Host mitochondria: more than an organelle in SARS-CoV-2 infection.Front Cell Infect Microbiol. 2023 Aug 25;13:1228275. doi: 10.3389/fcimb.2023.1228275. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37692170 Free PMC article. Review.
References
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. - PubMed
-
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

