Interplay of Obesity, Ethanol, and Contaminant Mixture on Clinical Profiles of Cardiovascular and Metabolic Diseases: Evidence from an Animal Study
- PMID: 35429258
- PMCID: PMC9107407
- DOI: 10.1007/s12012-022-09738-6
Interplay of Obesity, Ethanol, and Contaminant Mixture on Clinical Profiles of Cardiovascular and Metabolic Diseases: Evidence from an Animal Study
Abstract
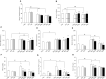
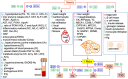
Obesity, ethanol, and contaminants are known risk factors of cardiovascular and metabolic diseases (CMD). However, their interplay on clinical profiles of these diseases remains unclear, and thus were investigated in this study. Male lean or obese JCR rats were given water or 10% ethanol and orally treated with or without a contaminant mixture (CM) dissolved in corn oil and loaded on two cookies at 0, 1.6, or 16 mg/kg BW/day dose levels for 4 weeks. The CM consisted 22 environmental contaminants found in human blood or serum of Northern populations. Over 60 parameters related to CMD were examined. The results revealed that obesity in JCR rats resembles the clinical profiles of non-alcoholic fatty liver disease in humans. Obesity was also associated with increased serum and organ retention of mercury, one of the chemical components of CM. Exposure to ethanol lightened hyperlipidemia, increased liver retention of mercury, and increased risk for hypertension in the obese rats. CM lessened hyperlipidemia and hyperenzymemia, worsened systemic inflammation and increased the risk for hypertension in the obese rats. CM markedly increased serum ethanol levels with or without ethanol exposure. Tissue total mercury contents significantly correlated with clinical parameters with altered profiles by both ethanol and obesity. These results suggest that obese individuals may be more prone to contaminant accumulation. Ethanol and CM exposure can alter clinical profiles associated with obesity, which may lead to misdiagnosis of CMD associated with obesity. CM can alter endogenous production and/or metabolism of ethanol, further complicating disease progression, diagnosis, and treatment.
Keywords: Cardiovascular and metabolic diseases; Clinical markers; Contaminants; Ethanol; Obesity.
© 2022. Crown.
Conflict of interest statement
There is no conflict of interest related to the work described in this manuscript.
Figures





References
-
- Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

