Baseline Characteristics and Treatment Response to Ixekizumab Categorised by Sex in Radiographic and Non-radiographic Axial Spondylarthritis Through 52 Weeks: Data from Three Phase III Randomised Controlled Trials
- PMID: 35429281
- PMCID: PMC9123018
- DOI: 10.1007/s12325-022-02132-2
Baseline Characteristics and Treatment Response to Ixekizumab Categorised by Sex in Radiographic and Non-radiographic Axial Spondylarthritis Through 52 Weeks: Data from Three Phase III Randomised Controlled Trials
Abstract
Objectives: Assess baseline characteristics and treatment response to ixekizumab (IXE) categorised by sex in patients with radiographic axial spondyloarthritis (r-axSpA) and non-radiographic axSpA (nr-axSpA) up to 52 weeks.
Methods: Data were analysed from three randomised controlled trials of IXE through 52 weeks. Patients fulfilled ASAS classification criteria for r-axSpA or nr-axSpA and were randomised to receive 80 mg subcutaneous administration of IXE every 2 weeks (Q2W) or 4 weeks (Q4W), or placebo (16 weeks COAST-V/W; 52 weeks COAST-X). Baseline characteristics and treatment outcomes were assessed. Patients were categorised by sex; methods included non-responder imputation for categorical variables, and modified baseline observation carried forward for continuous efficacy variables.
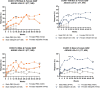
Results: At presentation, female patients had higher disease burden as reflected by significantly higher spinal pain at night, fatigue scores and pain/swelling in joints other than the neck, back or hip. ASAS40 response rate with the approved label dose, IXEQ4W, was achieved in 39% of male patients with r-axSpA by week 16, and 44% by week 52. For female patients, 16.7% and 33.3% achieved ASAS40 at week 16 and 52, respectively. In nr-axSpA, 46% of male patients achieved ASAS40 at week 16 and 30% at week 52. In total, 23.9% of female patients achieved ASAS40 at week 16, and 30.4% at week 52.
Conclusions: This analysis demonstrates that for the axSpA disease spectrum, female patients present with higher disease burden. Following treatment with IXE, there is a higher proportion of male responders up to 16 weeks, while female patients show less robust responses for the first 16 weeks but larger responses from weeks 16 through 52.
Trial registration numbers: NCT02696785, NCT02696798 and NCT02757352.
Keywords: Axial spondyloarthritis; Ixekizumab; Non-radiographic; Radiographic; Sex differences.
© 2022. The Author(s).
Figures

References
-
- Kennedy LG, Will R, Calin A. Sex ratio in the spondyloarthropathies and its relationship to phenotypic expression, mode of inheritance and age at onset. J Rheumatol. 1993;20(11):1900–1904. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

