Excitatory Crossmodal Input to a Widespread Population of Primary Sensory Cortical Neurons
- PMID: 35429324
- PMCID: PMC9554107
- DOI: 10.1007/s12264-022-00855-4
Excitatory Crossmodal Input to a Widespread Population of Primary Sensory Cortical Neurons
Abstract
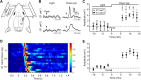
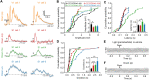
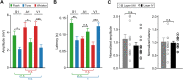
Crossmodal information processing in sensory cortices has been reported in sparsely distributed neurons under normal conditions and can undergo experience- or activity-induced plasticity. Given the potential role in brain function as indicated by previous reports, crossmodal connectivity in the sensory cortex needs to be further explored. Using perforated whole-cell recording in anesthetized adult rats, we found that almost all neurons recorded in the primary somatosensory, auditory, and visual cortices exhibited significant membrane-potential responses to crossmodal stimulation, as recorded when brain activity states were pharmacologically down-regulated in light anesthesia. These crossmodal cortical responses were excitatory and subthreshold, and further seemed to be relayed primarily by the sensory thalamus, but not the sensory cortex, of the stimulated modality. Our experiments indicate a sensory cortical presence of widespread excitatory crossmodal inputs, which might play roles in brain functions involving crossmodal information processing or plasticity.
Keywords: Crossmodal input; Crossmodal plasticity; Crossmodal response; Crossmodal task; GABAergic transmission; Sensory cortex; Sensory loss.
© 2022. Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests concerning the subject of this study.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources

