Recurrent Respiratory Syncytial Virus Infection in a CD14-Deficient Patient
- PMID: 35429403
- PMCID: PMC9400420
- DOI: 10.1093/infdis/jiac114
Recurrent Respiratory Syncytial Virus Infection in a CD14-Deficient Patient
Abstract
Background: Recurrent respiratory syncytial virus (RSV) infection requiring hospitalization is rare and the underlying mechanism is unknown. We aimed to determine the role of CD14-mediated immunity in the pathogenesis of recurrent RSV infection.
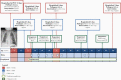
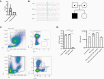
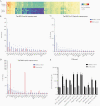
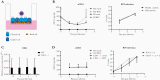
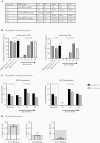
Methods: We performed genotyping and longitudinal immunophenotyping of the first patient with a genetic CD14 deficiency who developed recurrent RSV infection. We analyzed gene expression profiles and interleukin (IL)-6 production by patient peripheral blood mononuclear cells in response to RSV pre- and post-fusion (F) protein. We generated CD14-deficient human nasal epithelial cells cultured at air-liquid interface (HNEC-ALI) of patient-derived cells and after CRISPR-based gene editing of control cells. We analyzed viral replication upon RSV infection.
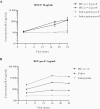
Results: Sanger sequencing revealed a homozygous single-nucleotide deletion in CD14, resulting in absence of the CD14 protein in the index patient. In vitro, viral replication was similar in wild-type and CD14-/- HNEC-ALI. Loss of immune cell CD14 led to impaired cytokine and chemokine responses to RSV pre- and post-F protein, characterized by absence of IL-6 production.
Conclusions: We report an association of recurrent RSV bronchiolitis with a loss of CD14 function in immune cells. Lack of CD14 function led to defective immune responses to RSV pre- and post-F protein without a change in viral replication.
Keywords: CD14; Toll-like receptor; epithelium; monocyte; respiratory syncytial virus.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
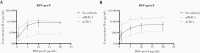
Comment in
-
CD14 and Related Genes in Respiratory Morbidity After Respiratory Syncytial Virus Infection.J Infect Dis. 2022 Sep 28;226(7):1295-1297. doi: 10.1093/infdis/jiac248. J Infect Dis. 2022. PMID: 35714332 No abstract available.
References
-
- Scheltema NM, Kavelaars XM, Thorburn K, et al. Potential impact of maternal vaccination on life-threatening respiratory syncytial virus infection during infancy. Vaccine 2018; 36:4693–700. - PubMed
-
- Crank MC, Ruckwardt TJ, Chen M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365:505–9. - PubMed
-
- Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. - PubMed
-
- Openshaw PJM, Chiu C, Culley FJ, Johansson C.. Protective and harmful immunity to RSV infection. Annu Rev Immunol 2017; 35:501–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

