Synthesis of 11-aminoalkoxy substituted benzophenanthridine derivatives as tyrosyl-DNA phosphodiesterase 1 inhibitors and their anticancer activity
- PMID: 35429714
- PMCID: PMC10557912
- DOI: 10.1016/j.bioorg.2022.105789
Synthesis of 11-aminoalkoxy substituted benzophenanthridine derivatives as tyrosyl-DNA phosphodiesterase 1 inhibitors and their anticancer activity
Abstract
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is an enzyme that repairs DNA lesions caused by the trapping of DNA topoisomerase IB (TOP1)-DNA break-associated crosslinks. TDP1 inhibitors have synergistic effect with TOP1 inhibitors in cancer cells and can overcome cancer cell resistance to TOP1 inhibitors. Here, we report the synthesis of 11-aminoalkoxy substituted benzophenanthridine derivatives as selective TDP1 inhibitors and show that six compounds 14, 16, 18, 20, 25 and 27 exhibit high TDP1 inhibition potency. The most potent TDP1 inhibitor 14 (IC50 = 1.7 ± 0.24 μM) induces cellular TDP1cc formation and shows synergistic effect with topotecan in four human cancer cell lines MCF-7, A549, H460 and HepG2.
Keywords: Anticancer; Benzophenanthridine; DNA repair; Topoisomerase; Tyrosyl-DNA phosphodiesterase.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
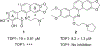



References
-
- Stewart L, Redinbo MR, Qiu X, Hol WGJ, Champoux JJ, A model for the mechanism of human topoisomerase I, Science 279 (5356) (1998) 1534–1541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

