Boosting maternal and neonatal humoral immunity following SARS-CoV-2 infection using a single messenger RNA vaccine dose
- PMID: 35430228
- PMCID: PMC9008977
- DOI: 10.1016/j.ajog.2022.04.010
Boosting maternal and neonatal humoral immunity following SARS-CoV-2 infection using a single messenger RNA vaccine dose
Abstract
Background: Post-COVID-19 vaccine boosting is a potent tool in the ongoing pandemic. Relevant data regarding this approach during pregnancy are lacking, which affects vaccination policy guidance, public acceptance, and vaccine uptake during pregnancy. We aimed to investigate the dynamics of anti-SARS-CoV-2 antibody levels following SARS-CoV-2 infection during pregnancy and to characterize the effect of a single postinfection vaccine booster dose on the anti-SARS-CoV-2 antibody levels in parturients in comparison with the levels in naïve vaccinated and convalescent, nonboosted parturients.
Study design: Serum samples prospectively collected from parturients and umbilical cords at delivery at our university-affiliated urban medical center in Jerusalem, Israel, from May to October 2021, were selected and analyzed in a case-control manner. Study groups comprised the following participants: a consecutive sample of parturients with a polymerase chain reaction-confirmed history of COVID-19 during any stage of pregnancy; and comparison groups selected according to time of exposure comprising (1) convalescent, nonboosted parturients with polymerase chain reaction-confirmed COVID-19; (2) convalescent parturients with polymerase chain reaction-confirmed COVID-19 who received a single booster dose of the BNT162b2 messenger RNA vaccine; and (3) infection-naïve, fully vaccinated parturients who received 2 doses of the BNT162b2 messenger RNA vaccine. Outcomes that were determined included maternal and umbilical cord blood anti-SARS-CoV-2 antibody levels detected at delivery, the reported side effects, and pregnancy outcomes.
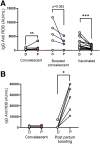
Results: A total of 228 parturients aged 18 to 45 years were included. Of those, samples from 64 were studied to characterize the titer dynamics following COVID-19 at all stages of pregnancy. The boosting effect was determined by comparing (1) convalescent (n=54), (2) boosted convalescent (n=60), and (3) naïve, fully vaccinated (n=114) parturients. Anti-SARS-CoV-2 antibody levels detected on delivery showed a gradual and significant decline over time from infection to delivery (r=0.4371; P=.0003). Of the gravidae infected during the first trimester, 34.6% (9/26) tested negative at delivery, compared with 9.1% (3/33) of those infected during the second trimester (P=.023). Significantly higher anti-SARS-CoV-2 antibody levels were observed among boosted convalescent than among nonboosted convalescent (17.6-fold; P<.001) and naïve vaccinated parturients (3.2-fold; P<.001). Similar patterns were observed in umbilical cord blood. Side effects in convalescent gravidae resembled those in previous reports of mild symptoms following COVID-19 vaccination during pregnancy.
Conclusion: Postinfection maternal humoral immunity wanes during pregnancy, leading to low or undetectable protective titers for a marked proportion of patients. A single boosting dose of the BNT162b2 messenger RNA vaccine induced a robust increase in protective titers for both the mother and newborn with moderate reported side effects.
Keywords: COVID-19; anti SARS-CoV-2 antibody; boosting vaccine dose; case-control study; postinfection vaccine; pregnancy; single dose; vaccine during.
Copyright © 2022. Published by Elsevier Inc.
Figures





References
-
- Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596:276–280. - PubMed
-
- Abbasi J. Study suggests lasting immunity after COVID-19, with a big boost from vaccination. JAMA. 2021;326:376–377. - PubMed
-
- Del Rio C., Omer S.B., Malani P.N. Winter of Omicron-the evolving COVID-19 pandemic. JAMA. 2022;327:319–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

