Cellular mechanism of action of 2-nitroimidazoles as hypoxia-selective therapeutic agents
- PMID: 35430547
- PMCID: PMC9038562
- DOI: 10.1016/j.redox.2022.102300
Cellular mechanism of action of 2-nitroimidazoles as hypoxia-selective therapeutic agents
Abstract
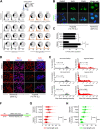
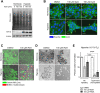
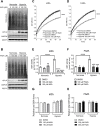
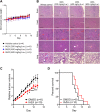
Solid tumours are often poorly oxygenated, which confers resistance to standard treatment modalities. Targeting hypoxic tumours requires compounds, such as nitroimidazoles (NIs), equipped with the ability to reach and become activated within diffusion limited tumour niches. NIs become selectively entrapped in hypoxic cells through bioreductive activation, and have shown promise as hypoxia directed therapeutics. However, little is known about their mechanism of action, hindering the broader clinical usage of NIs. Iodoazomycin arabinofuranoside (IAZA) and fluoroazomycin arabinofuranoside (FAZA) are clinically validated 2-NI hypoxic radiotracers with excellent tumour uptake properties. Hypoxic cancer cells have also shown preferential susceptibility to IAZA and FAZA treatment, making them ideal candidates for an in-depth study in a therapeutic setting. Using a head and neck cancer model, we show that hypoxic cells display higher sensitivity to IAZA and FAZA, where the drugs alter cell morphology, compromise DNA replication, slow down cell cycle progression and induce replication stress, ultimately leading to cytostasis. Effects of IAZA and FAZA on target cellular macromolecules (DNA, proteins and glutathione) were characterized to uncover potential mechanism(s) of action. Covalent binding of these NIs was only observed to cellular proteins, but not to DNA, under hypoxia. While protein levels remained unaffected, catalytic activities of NI target proteins, such as the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the detoxification enzyme glutathione S-transferase (GST) were significantly curtailed in response to drug treatment under hypoxia. Intraperitoneal administration of IAZA was well-tolerated in mice and produced early (but transient) growth inhibition of subcutaneous mouse tumours.
Keywords: Head and neck tumour; Hypoxia; Nitroimidazole; Replication stress.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Brown J.M. The hypoxic cell A target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1999;59(23):5863–5870. - PubMed
-
- Ai M., et al. Tumor hypoxia drives immune suppression and immunotherapy resistance. J. Immunother. Cancer. 2015;3(2) 1-1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

