The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids
- PMID: 35430804
- PMCID: PMC9013454
- DOI: 10.1186/s40168-022-01255-6
The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids
Erratum in
-
Correction to: The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids.Microbiome. 2022 May 4;10(1):70. doi: 10.1186/s40168-022-01277-0. Microbiome. 2022. PMID: 35509035 Free PMC article. No abstract available.
-
Correction: The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids.Microbiome. 2024 Jun 4;12(1):100. doi: 10.1186/s40168-024-01842-9. Microbiome. 2024. PMID: 38835074 Free PMC article. No abstract available.
Abstract
Background: Chronic cerebral hypoperfusion (CCH) underlies secondary brain injury following certain metabolic disorders and central nervous system (CNS) diseases. Dysregulation of the microbiota-gut-brain axis can exacerbate various CNS disorders through aberrantly expressed metabolites such as short-chain fatty acids (SCFAs). Yet, its relationship with CCH remains to be demonstrated. And if so, it is of interest to explore whether restoring gut microbiota to maintain SCFA metabolism could protect against CCH.
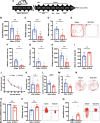
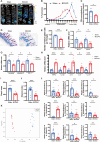
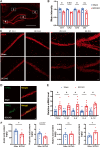
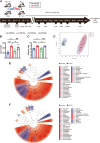
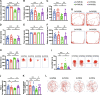
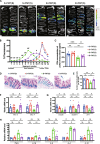
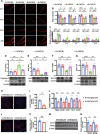
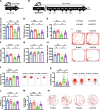
Results: Rats subjected to bilateral common carotid artery occlusion (BCCAO) as a model of CCH exhibited cognitive impairment, depressive-like behaviors, decreased gut motility, and compromised gut barrier functions. The 16S ribosomal RNA gene sequencing revealed an abnormal gut microbiota profile and decreased relative abundance of some representative SCFA producers, with the decreased hippocampal SCFAs as the further evidence. Using fecal microbiota transplantation (FMT), rats recolonized with a balanced gut microbiome acquired a higher level of hippocampal SCFAs, as well as decreased neuroinflammation when exposed to lipopolysaccharide. Healthy FMT promoted gut motility and gut barrier functions, and improved cognitive decline and depressive-like behaviors by inhibiting hippocampal neuronal apoptosis in BCCAO rats. Long-term SCFA supplementation further confirmed its neuroprotective effect in terms of relieving inflammatory response and hippocampal neuronal apoptosis following BCCAO.
Conclusion: Our results demonstrate that modulating the gut microbiome via FMT can ameliorate BCCAO-induced gut dysbiosis, cognitive decline, and depressive-like behaviors, possibly by enhancing the relative abundance of SCFA-producing floras and subsequently increasing SCFA levels. Video abstract.
Keywords: Chronic cerebral hypoperfusion; Fecal microbiota transplantation; Gut dysbiosis; Gut microbiota; Short-chain fatty acids.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–S9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

