Human Coronary Plaque T Cells Are Clonal and Cross-React to Virus and Self
- PMID: 35430876
- PMCID: PMC9286288
- DOI: 10.1161/CIRCRESAHA.121.320090
Human Coronary Plaque T Cells Are Clonal and Cross-React to Virus and Self
Abstract
Background: Coronary artery disease is an incurable, life-threatening disease that was once considered primarily a disorder of lipid deposition. Coronary artery disease is now also characterized by chronic inflammation' notable for the buildup of atherosclerotic plaques containing immune cells in various states of activation and differentiation. Understanding how these immune cells contribute to disease progression may lead to the development of novel therapeutic strategies.
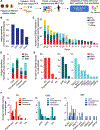
Methods: We used single-cell technology and in vitro assays to interrogate the immune microenvironment of human coronary atherosclerotic plaque at different stages of maturity.
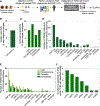
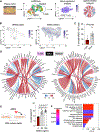
Results: In addition to macrophages, we found a high proportion of αβ T cells in the coronary plaques. Most of these T cells lack high expression of CCR7 and L-selectin, indicating that they are primarily antigen-experienced memory cells. Notably, nearly one-third of these cells express the HLA-DRA surface marker, signifying activation through their TCRs (T-cell receptors). Consistent with this, TCR repertoire analysis confirmed the presence of activated αβ T cells (CD4<CD8), exhibiting clonal expansion of specific TCRs. Interestingly, we found that these plaque T cells had TCRs specific for influenza, coronavirus, and other viral epitopes, which share sequence homologies to proteins found on smooth muscle cells and endothelial cells, suggesting potential autoimmune-mediated T-cell activation in the absence of active infection. To better understand the potential function of these activated plaque T cells, we then interrogated their transcriptome at the single-cell level. Of the 3 T-cell phenotypic clusters with the highest expression of the activation marker HLA-DRA, 2 clusters expressed a proinflammatory and cytolytic signature characteristic of CD8 cells, while the other expressed AREG (amphiregulin), which promotes smooth muscle cell proliferation and fibrosis, and, thus, contributes to plaque progression.
Conclusions: Taken together, these findings demonstrate that plaque T cells are clonally expanded potentially by antigen engagement, are potentially reactive to self-epitopes, and may interact with smooth muscle cells and macrophages in the plaque microenvironment.
Keywords: T-lymphocytes; atherosclerosis; coronary artery disease; endothelial cells; humans; plaque, atherosclerotic.
Conflict of interest statement
DISCLOSURES
The authors have no conflicts to disclose.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

