Optimal Detection of Latent Mycobacterium tuberculosis Infection by Combined Heparin-Binding Hemagglutinin (HBHA) and Early Secreted Antigenic Target 6 (ESAT-6) Whole-Blood Interferon Gamma Release Assays
- PMID: 35430897
- PMCID: PMC9116186
- DOI: 10.1128/jcm.02443-21
Optimal Detection of Latent Mycobacterium tuberculosis Infection by Combined Heparin-Binding Hemagglutinin (HBHA) and Early Secreted Antigenic Target 6 (ESAT-6) Whole-Blood Interferon Gamma Release Assays
Abstract
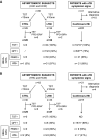
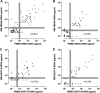
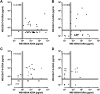
Optimal detection of latent tuberculosis (TB) infection (LTBI) remains a challenge, although it is essential to reach the goal of TB elimination. Our objective was to develop and clinically evaluate a user-friendly, 24-h, whole-blood (WB) interferon gamma (IFN-γ) release assay (IGRA) improving the detection of LTBI, compared to available tests. One milliliter of blood was divided into four aliquots and in vitro stimulated for 24 h with two different stage-specific mycobacterial antigens, i.e., heparin-binding hemagglutinin (HBHA) and early secreted antigenic target 6 (ESAT-6), a latency-associated antigen and a bacterial replication-related antigen, respectively, in addition to positive and negative controls. Clinical evaluation was performed on two independent cohorts of carefully selected subjects, i.e., a training cohort of 83 individuals and a validation cohort of 69 individuals. Both cohorts comprised LTBI subjects (asymptomatic people with a positive tuberculin skin test result and potential exposure to TB index cases), patients with active TB (aTB), and noninfected controls. The sensitivity and specificity of the WB-HBHA-IGRA to identify LTBI subjects among asymptomatic individuals were 93%. Combining the results in response to HBHA and ESAT-6 allowed us to identify LTBI subgroups. One group, with IFN-γ responses to HBHA only, was easily differentiated from patients with aTB. The other group, responding to both antigens like the aTB group, is likely at risk to reactivate the infection and should be prioritized for prophylactic anti-TB treatment. The combined WB-IGRA may be offered to clinicians for the selection of LTBI subjects to benefit from prophylactic treatment.
Keywords: early secreted antigenic target 6; heparin-binding hemagglutinin; interferon gamma release assay; latent tuberculosis infection; whole blood.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Immuno-Diagnosis of Active Tuberculosis by a Combination of Cytokines/Chemokines Induced by Two Stage-Specific Mycobacterial Antigens: A Pilot Study in a Low TB Incidence Country.Front Immunol. 2022 Mar 10;13:842604. doi: 10.3389/fimmu.2022.842604. eCollection 2022. Front Immunol. 2022. PMID: 35359958 Free PMC article.
-
Mycobacterial heparin-binding hemagglutinin (HBHA)-induced interferon-γ release assay (IGRA) for discrimination of latent and active tuberculosis: A systematic review and meta-analysis.PLoS One. 2021 Jul 16;16(7):e0254571. doi: 10.1371/journal.pone.0254571. eCollection 2021. PLoS One. 2021. PMID: 34270559 Free PMC article.
-
Risk stratification of latent tuberculosis defined by combined interferon gamma release assays.PLoS One. 2012;7(8):e43285. doi: 10.1371/journal.pone.0043285. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912846 Free PMC article.
-
QuantiFERON-TB Gold Plus combined with HBHA-Induced IFN-γ release assay improves the accuracy of identifying tuberculosis disease status.Tuberculosis (Edinb). 2020 Sep;124:101966. doi: 10.1016/j.tube.2020.101966. Epub 2020 Aug 6. Tuberculosis (Edinb). 2020. PMID: 32866942
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
Cited by
-
Mycobacterium tuberculosis: The Mechanism of Pathogenicity, Immune Responses, and Diagnostic Challenges.J Clin Lab Anal. 2024 Dec;38(23):e25122. doi: 10.1002/jcla.25122. Epub 2024 Nov 26. J Clin Lab Anal. 2024. PMID: 39593272 Free PMC article. Review.
-
Predictive biomarkers for latent Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2024 Jul;147:102399. doi: 10.1016/j.tube.2023.102399. Epub 2023 Aug 24. Tuberculosis (Edinb). 2024. PMID: 37648595 Free PMC article.
-
Immuno-Diagnosis of Active Tuberculosis by a Combination of Cytokines/Chemokines Induced by Two Stage-Specific Mycobacterial Antigens: A Pilot Study in a Low TB Incidence Country.Front Immunol. 2022 Mar 10;13:842604. doi: 10.3389/fimmu.2022.842604. eCollection 2022. Front Immunol. 2022. PMID: 35359958 Free PMC article.
-
Global, regional, and national burden of HIV-negative tuberculosis, 1990-2021: findings from the Global Burden of Disease Study 2021.Infect Dis Poverty. 2024 Aug 19;13(1):60. doi: 10.1186/s40249-024-01227-y. Infect Dis Poverty. 2024. PMID: 39155365 Free PMC article.
-
Analysis of a Combined HBHA and ESAT-6-Interferon-γ-Release Assay for the Diagnosis of Tuberculous Lymphadenopathies.J Clin Med. 2023 Mar 8;12(6):2127. doi: 10.3390/jcm12062127. J Clin Med. 2023. PMID: 36983128 Free PMC article.
References
-
- World Health Organization. 2021. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa....
-
- Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B, Arend SM, Detjen A, Bothamley G, Zellweger JP, Milburn H, Diel R, Ravn P, Cobelens F, Cardona PJ, Kan B, Solovic I, Duarte R, Cirillo DM, Lange C, TBNET. 2009. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 33:956–973. doi:10.1183/09031936.00120908. - DOI - PubMed
-
- Della Bella C, Spinicci M, Alnwaisri HFM, Bartalesi F, Tapinassi S, Mencarini J, Benagiano M, Grassi A, D'Elios S, Troilo A, Abilbayeva A, Kuashova D, Bitanova E, Tarabayeva A, Shuralev EA, Bartoloni A, D'Elios MM. 2020. LIOFeron®TB/LTBI: a novel and reliable test for LTBI and tuberculosis. Int J Infect Dis 91:177–181. doi:10.1016/j.ijid.2019.12.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

