Reversible Addition-Fragmentation Chain Transfer Aqueous Dispersion Polymerization of 4-Hydroxybutyl Acrylate Produces Highly Thermoresponsive Diblock Copolymer Nano-Objects
- PMID: 35431331
- PMCID: PMC9007527
- DOI: 10.1021/acs.macromol.1c02431
Reversible Addition-Fragmentation Chain Transfer Aqueous Dispersion Polymerization of 4-Hydroxybutyl Acrylate Produces Highly Thermoresponsive Diblock Copolymer Nano-Objects
Abstract
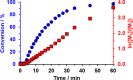
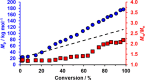
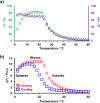
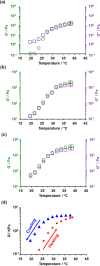
The reversible addition-fragmentation chain transfer (RAFT) aqueous dispersion polymerization of 2-hydroxypropyl methacrylate (HPMA) using a poly(glycerol monomethacrylate) (PGMA) precursor is an important prototypical example of polymerization-induced self-assembly. 4-Hydroxybutyl acrylate (HBA) is a structural isomer of HPMA, but the former monomer exhibits appreciably higher aqueous solubility. For the two corresponding homopolymers, PHBA is more weakly hydrophobic than PHPMA. Moreover, PHBA has a significantly lower glass transition temperature (T g) so it exhibits much higher chain mobility than PHPMA at around ambient temperature. In view of these striking differences, we have examined the RAFT aqueous dispersion polymerization of HBA using a PGMA precursor with the aim of producing a series of PGMA57-300-PHBA100-1580 diblock copolymer nano-objects by systematic variation of the mean degree of polymerization of each block. A pseudo-phase diagram is constructed using transmission electron microscopy to assign the copolymer morphology after employing glutaraldehyde to cross-link the PHBA chains and hence prevent film formation during grid preparation. The thermoresponsive character of the as-synthesized linear nano-objects is explored using dynamic light scattering and temperature-dependent rheological measurements. Comparison with the analogous PGMA x -PHPMA y formulation is made where appropriate. In particular, we demonstrate that replacing the structure-directing PHPMA block with PHBA leads to significantly greater thermoresponsive behavior over a much wider range of diblock copolymer compositions. Given that PGMA-PHPMA worm gels can induce stasis in human stem cells (see Canton et al., ACS Central Science, 2016, 2, 65-74), our findings are likely to have implications for the design of next-generation PGMA-PHBA worm gels for cell biology applications.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Synthesis of Thermoresponsive Diblock Copolymer Nano-Objects via RAFT Aqueous Emulsion Polymerization of Hydroxybutyl Methacrylate.Macromolecules. 2022 Apr 26;55(8):3051-3062. doi: 10.1021/acs.macromol.2c00379. Epub 2022 Apr 17. Macromolecules. 2022. PMID: 35492576 Free PMC article.
-
Shape-shifting thermoreversible diblock copolymer nano-objects via RAFT aqueous dispersion polymerization of 4-hydroxybutyl acrylate.Chem Sci. 2021 Oct 7;12(41):13719-13729. doi: 10.1039/d1sc05022b. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760156 Free PMC article.
-
Critical Dependence of Molecular Weight on Thermoresponsive Behavior of Diblock Copolymer Worm Gels in Aqueous Solution.Macromolecules. 2018 Nov 13;51(21):8357-8371. doi: 10.1021/acs.macromol.8b01617. Epub 2018 Oct 16. Macromolecules. 2018. PMID: 30449901 Free PMC article.
-
Polymerization-induced self-assembly and disassembly during the synthesis of thermoresponsive ABC triblock copolymer nano-objects in aqueous solution.Chem Sci. 2022 Jun 8;13(24):7295-7303. doi: 10.1039/d2sc01611g. eCollection 2022 Jun 22. Chem Sci. 2022. PMID: 35799807 Free PMC article.
-
Shape-Shifting Thermoresponsive Block Copolymer Nano-Objects.J Colloid Interface Sci. 2023 Mar 15;634:906-920. doi: 10.1016/j.jcis.2022.12.080. Epub 2022 Dec 18. J Colloid Interface Sci. 2023. PMID: 36566636 Review.
Cited by
-
Polymerization-Induced Self-Assembly for Efficient Fabrication of Biomedical Nanoplatforms.Research (Wash D C). 2023 Apr 11;6:0113. doi: 10.34133/research.0113. eCollection 2023. Research (Wash D C). 2023. PMID: 37223484 Free PMC article. Review.
-
Synthesis and Characterization of Charge-Stabilized Poly(4-hydroxybutyl acrylate) Latex by RAFT Aqueous Dispersion Polymerization: A New Precursor for Reverse Sequence Polymerization-Induced Self-Assembly.Macromolecules. 2023 Jun 2;56(11):4296-4306. doi: 10.1021/acs.macromol.3c00534. eCollection 2023 Jun 13. Macromolecules. 2023. PMID: 37333840 Free PMC article.
-
Block copolymer synthesis in ionic liquid via polymerisation-induced self-assembly: a convenient route to gel electrolytes.Chem Sci. 2024 Feb 13;15(12):4416-4426. doi: 10.1039/d3sc06717c. eCollection 2024 Mar 20. Chem Sci. 2024. PMID: 38516087 Free PMC article.
-
Preparation of diblock copolymer nano-assemblies by ultrasonics assisted ethanol-phase polymerization-induced self-assembly.Ultrason Sonochem. 2024 May;105:106855. doi: 10.1016/j.ultsonch.2024.106855. Epub 2024 Mar 23. Ultrason Sonochem. 2024. PMID: 38531733 Free PMC article.
-
Synthesis of Thermoresponsive Diblock Copolymer Nano-Objects via RAFT Aqueous Emulsion Polymerization of Hydroxybutyl Methacrylate.Macromolecules. 2022 Apr 26;55(8):3051-3062. doi: 10.1021/acs.macromol.2c00379. Epub 2022 Apr 17. Macromolecules. 2022. PMID: 35492576 Free PMC article.
References
-
- Charleux B.; Delaittre G.; Rieger J.; D’Agosto F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. 10.1021/ma300713f. - DOI
-
- Wang G.; Schmitt M.; Wang Z.; Lee B.; Pan X.; Fu L.; Yan J.; Li S.; Xie G.; Bockstaller M. R.; Matyjaszewski K. Polymerization-Induced Self-Assembly (PISA) Using ICAR ATRP at Low Catalyst Concentration. Macromolecules 2016, 49, 8605–8615. 10.1021/acs.macromol.6b01966. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
