Selectivity, Speciation, and Substrate Control in the Gold-Catalyzed Coupling of Indoles and Alkynes
- PMID: 35431397
- PMCID: PMC9007570
- DOI: 10.1021/acs.organomet.2c00035
Selectivity, Speciation, and Substrate Control in the Gold-Catalyzed Coupling of Indoles and Alkynes
Abstract
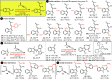
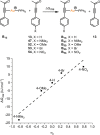
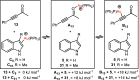
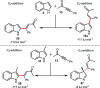
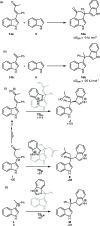
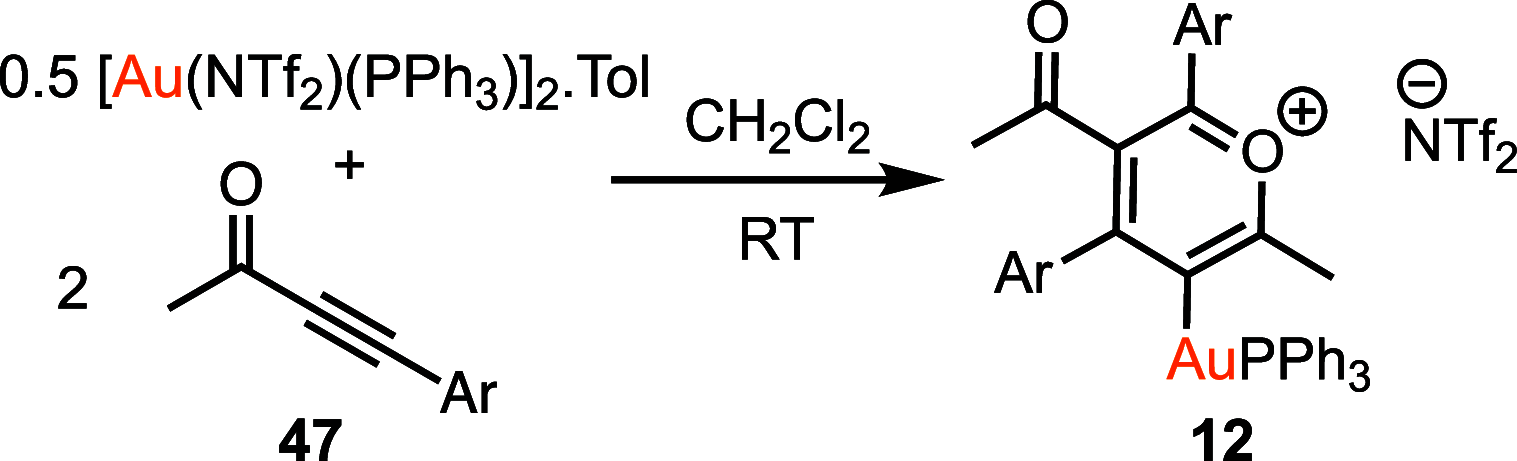
A convenient and mild protocol for the gold-catalyzed intermolecular coupling of substituted indoles with carbonyl-functionalized alkynes to give vinyl indoles is reported. This reaction affords 3-substituted indoles in high yield, and in contrast to the analogous reactions with simple alkynes which give bisindolemethanes, only a single indole is added to the alkyne. The protocol is robust and tolerates substitution at a range of positions of the indole and the use of ester-, amide-, and ketone-substituted alkynes. The use of 3-substituted indoles as substrates results in the introduction of the vinyl substituent at the 2-position of the ring. A combined experimental and computational mechanistic study has revealed that the gold catalyst has a greater affinity to the indole than the alkyne, despite the carbon-carbon bond formation step proceeding through an η2(π)-alkyne complex, which helps to explain the stark differences between the intra- and intermolecular variants of the reaction. This study also demonstrated that the addition of a second indole to the carbonyl-containing vinyl indole products is both kinetically and thermodynamically less favored than in the case of more simple alkynes, providing an explanation for the observed selectivity. Finally, a highly unusual gold-promoted alkyne dimerization reaction to form a substituted gold pyrylium salt has been identified and studied in detail.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Intra- and intermolecular reactions of indoles with alkynes catalyzed by gold.Chemistry. 2007;13(5):1358-73. doi: 10.1002/chem.200601324. Chemistry. 2007. PMID: 17206736
-
Assembly of conjugated enynes and substituted indoles via CuI/amino acid-catalyzed coupling of 1-alkynes with vinyl iodides and 2-bromotrifluoroacetanilides.J Org Chem. 2007 Jun 22;72(13):4844-50. doi: 10.1021/jo070547x. Epub 2007 May 27. J Org Chem. 2007. PMID: 17530901
-
Base-Mediated Hydroamination of Alkynes.Acc Chem Res. 2017 Feb 21;50(2):240-254. doi: 10.1021/acs.accounts.6b00449. Epub 2017 Jan 27. Acc Chem Res. 2017. PMID: 28128923
-
Azido-Alkynes in Gold(I)-Catalyzed Indole Syntheses.Chem Rec. 2021 Dec;21(12):3897-3910. doi: 10.1002/tcr.202100202. Epub 2021 Sep 8. Chem Rec. 2021. PMID: 34498385 Review.
-
Gold-catalyzed fluorination of alkynes/allenes: mechanistic explanations and reaction scope.Org Biomol Chem. 2024 Dec 18;23(1):11-35. doi: 10.1039/d4ob01579g. Org Biomol Chem. 2024. PMID: 39513472 Review.
Cited by
-
Advances in gold catalyzed synthesis of quinoid heteroaryls.RSC Adv. 2024 Jul 3;14(29):21047-21064. doi: 10.1039/d4ra03368j. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38962094 Free PMC article. Review.
-
Ligand effects, solvent cooperation, and large kinetic solvent deuterium isotope effects in gold(I)-catalyzed intramolecular alkene hydroamination.Beilstein J Org Chem. 2024 Feb 29;20:479-496. doi: 10.3762/bjoc.20.43. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38440168 Free PMC article.
-
Regiodivergent Gold-Catalyzed Rearrangement-Addition Reactions of Sulfenylated Propargylic Carboxylates with Indoles.Org Lett. 2024 Sep 13;26(36):7713-7717. doi: 10.1021/acs.orglett.4c02853. Epub 2024 Aug 29. Org Lett. 2024. PMID: 39207898 Free PMC article.
References
-
- Liddon J. T. R.; Rossi-Ashton J. A.; Clarke A. K.; Lynam J. M.; Taylor R. J. K.; Unsworth W. P. Divergent reactivity of indole-tethered ynones with silver(I) and gold(I) catalysts: A combined synthetic and computational study. Synthesis 2018, 50, 4829–4836. 10.1055/s-0037-1610181. - DOI
-
- James M. J.; Clubley R. E.; Palate K. Y.; Procter T. J.; Wyton A. C.; O’Brien P.; Taylor R. J. K.; Unsworth W. P. Silver(I)-catalyzed dearomatization of alkyne-tethered indoles: Divergent synthesis of spirocyclic indolenines and carbazoles. Org. Lett. 2015, 17, 4372–4375. 10.1021/acs.orglett.5b02216. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
