A Comparative Review of Pyroptosis in Mammals and Fish
- PMID: 35431566
- PMCID: PMC9012342
- DOI: 10.2147/JIR.S361266
A Comparative Review of Pyroptosis in Mammals and Fish
Abstract
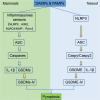
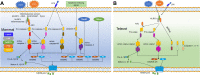
Pyroptosis is a form of programmed cell death, which is executed by gasdermin family proteins. Under the stimulation of pathogen- and/or damage-associated molecular patterns, pattern recognition receptors (PRRs) such as Nod like receptors could recruit apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspases to form inflammasomes and then activate caspases through various pathways. The activated caspases then cleave gasdermin family proteins, and N-terminal (NT) domains of gasdermins were released to form oligomeric pores, resulting in the increased membrane permeability, cell swelling, and final pyroptosis. During this process, caspases also promote the maturation and release of inflammatory cytokines such as IL-1β and IL-18, thus pyroptosis is also named inflammatory cell death. Unlike numerous gasdermin family proteins in mammals, only gasdermin E (GSDME) has been identified in fish. GSDME in fish can be cleaved by caspase-a/-b to release its NT domain and induce pyroptosis. Studies indicated that pyroptosis in fish mainly depends on NLR family pyrin domain-containing 3 (NLRP3) inflammasome. ASC and different caspase proteins also were identified in different fish species. The influences of pathogenic microorganism infection and environmental pollutants on fish pyroptosis were studied in recent years. Considering that fish living environment is affected by multiple factors such as water salinity, temperature, oxygen supply, and highly fluctuating food supply, the in-depth research about fish pyroptosis will contribute to revealing the mechanism of pyroptosis during evolution.
Keywords: caspases; fish; gasdermin E; pyroptosis.
© 2022 Song et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis.J Biol Chem. 2020 Jan 24;295(4):1120-1141. doi: 10.1074/jbc.RA119.011751. Epub 2019 Dec 18. J Biol Chem. 2020. PMID: 31852739 Free PMC article.
-
ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages.Apoptosis. 2019 Oct;24(9-10):703-717. doi: 10.1007/s10495-019-01551-x. Apoptosis. 2019. PMID: 31175486
-
The molecular mechanism and evolutionary divergence of caspase 3/7-regulated gasdermin E activation.Elife. 2024 Mar 15;12:RP89974. doi: 10.7554/eLife.89974. Elife. 2024. PMID: 38489483 Free PMC article.
-
Inflammasomes in Teleosts: Structures and Mechanisms That Induce Pyroptosis during Bacterial Infection.Int J Mol Sci. 2021 Apr 22;22(9):4389. doi: 10.3390/ijms22094389. Int J Mol Sci. 2021. PMID: 33922312 Free PMC article. Review.
-
Pollutant-induced pyroptosis in humans and other animals.Life Sci. 2023 Mar 1;316:121386. doi: 10.1016/j.lfs.2023.121386. Epub 2023 Jan 16. Life Sci. 2023. PMID: 36657639 Review.
Cited by
-
Identification of Haplotypes Associated With Resistance to Bacterial Cold Water Disease in Rainbow Trout Using Whole-Genome Resequencing.Front Genet. 2022 Jun 23;13:936806. doi: 10.3389/fgene.2022.936806. eCollection 2022. Front Genet. 2022. PMID: 35812729 Free PMC article.
-
The role of pyroptosis and its crosstalk with immune therapy in breast cancer.Front Immunol. 2022 Aug 30;13:973935. doi: 10.3389/fimmu.2022.973935. eCollection 2022. Front Immunol. 2022. PMID: 36119049 Free PMC article. Review.
-
Largemouth bass (Micropterus salmoides) exhibited better growth potential after adaptation to dietary cottonseed protein concentrate inclusion but experienced higher inflammatory risk during bacterial infection.Front Immunol. 2022 Sep 15;13:997985. doi: 10.3389/fimmu.2022.997985. eCollection 2022. Front Immunol. 2022. PMID: 36189250 Free PMC article.
-
The Evolution of NLR Inflammasome and Its Mediated Pyroptosis in Metazoa.Int J Mol Sci. 2024 Oct 17;25(20):11167. doi: 10.3390/ijms252011167. Int J Mol Sci. 2024. PMID: 39456947 Free PMC article.
-
CcGSDMEa functions the pore-formation in cytomembrane and the regulation on the secretion of IL-lβ in common carp (Cyprinus carpio haematopterus).Front Immunol. 2023 Jan 6;13:1110322. doi: 10.3389/fimmu.2022.1110322. eCollection 2022. Front Immunol. 2023. PMID: 36685536 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

