Serum Phosphorus and Pill Burden Among Hemodialysis Patients Prescribed Sucroferric Oxyhydroxide: One-Year Follow-Up on a Contemporary Cohort
- PMID: 35431567
- PMCID: PMC9012313
- DOI: 10.2147/IJNRD.S353213
Serum Phosphorus and Pill Burden Among Hemodialysis Patients Prescribed Sucroferric Oxyhydroxide: One-Year Follow-Up on a Contemporary Cohort
Abstract
Purpose: In prior analyses of real-world cohorts of hemodialysis patients switched from one phosphate binder (PB) to sucroferric oxyhydroxide (SO), SO therapy has been associated with improvements in serum phosphorus (sP) and reductions in daily PB pill burden. To characterize how SO initiation patterns have changed over time, we examined the long-term effectiveness of SO in a contemporary (2018-2019) cohort.
Patients and methods: Adult Fresenius Kidney Care hemodialysis patients first prescribed SO monotherapy as part of routine care between May 2018 and May 2019 (N = 1792) were followed for 1 year. All patients received a non-SO PB during a 91-day baseline period before SO prescription. Mean PB pills/day and laboratory parameters were compared before and during SO treatment. Results were divided into consecutive 91-day intervals (Q1-Q4) and analyzed using linear mixed-effects regression and Cochran's Q test. These results were contrasted with findings from a historical (2014-2015) cohort (N = 530).
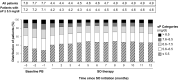
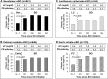
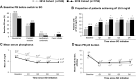
Results: The proportion of patients achieving sP ≤5.5 mg/dl increased after switching to SO (from 27.0% at baseline to 37.8%, 45.1%, 44.7%, and 44.0% at Q1, Q2, Q3, and Q4, respectively; P < 0.0001 for all). The mean daily PB pill burden decreased from a baseline of 7.7 to 4.4, 4.6, 4.8, and 4.9, respectively, across quarters (P < 0.0001 for all). Patients in the contemporary cohort had improved sP control (27.0% achieving sP ≤5.5 mg/dl vs 17.7%) and lower daily PB pill burden (mean 7.7 vs 8.5 pills/day) at baseline than those in the historical cohort. Overall use of active vitamin D was similar between cohorts, although higher use of oral active vitamin D (63.9% vs 15.7%) and lower use of IV active vitamin D lower (23.4% vs 74.2%) was observed in the contemporary cohort.
Conclusion: Despite evolving treatment patterns, switching to SO resulted in improved sP control with fewer pills per day in this contemporary hemodialysis cohort.
Keywords: hemodialysis; phosphate binder; pill burden; serum phosphorus; sucroferric oxyhydroxide.
© 2022 Kendrick et al.
Conflict of interest statement
JBK has participated in advisory boards for Fresenius Medical Care Renal Therapies Group, LLC. MZ, LHF, VP, CM, and MSA are employees of Fresenius Medical Care. CM owns stock in Fresenius Medical Care AG & Co. KGaA. DWC is a consultant for Fresenius Medical Care Renal Therapies Group, LLC, and a consultant for Akebia, GSK, AstraZeneca, FibroGen, Otsuka, and MediBeacon. The authors report no other conflicts of interest in this work.
Figures
References
-
- Sprague SM, Marcuccilli M, Rakov V. Clinical rationale of sucroferric oxyhydroxide for controlling hyperphosphatemia in patients with chronic kidney disease. Clin Investig (Lond). 2015;5(1):9–21. doi:10.4155/CLI.14.110 - DOI
LinkOut - more resources
Full Text Sources

