Heterogeneous CaMKII-Dependent Synaptic Compensations in CA1 Pyramidal Neurons From Acute Hippocampal Slices
- PMID: 35431809
- PMCID: PMC9005847
- DOI: 10.3389/fncel.2022.821088
Heterogeneous CaMKII-Dependent Synaptic Compensations in CA1 Pyramidal Neurons From Acute Hippocampal Slices
Abstract
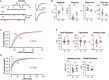
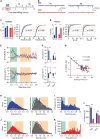
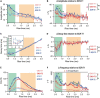
Prolonged changes in neural activity trigger homeostatic synaptic plasticity (HSP) allowing neuronal networks to operate within functional ranges. Cell-wide or input-specific adaptations can be induced by pharmacological or genetic manipulations of activity, and by sensory deprivation. Reactive functional changes caused by deafferentation may partially share mechanisms with HSP. Acute hippocampal slices are a suitable model to investigate relatively rapid (hours) modifications occurring after denervation and explore the underlying mechanisms. As during slicing many afferents are cut, we conducted whole-cell recordings of miniature excitatory postsynaptic currents (mEPSCs) in CA1 pyramidal neurons to evaluate changes over the following 12 h. As Schaffer collaterals constitute a major glutamatergic input to these neurons, we also dissected CA3. We observed an average increment in mEPSCs amplitude and a decrease in decay time, suggesting synaptic AMPA receptor upregulation and subunit content modifications. Sorting mEPSC by rise time, a correlate of synapse location along dendrites, revealed amplitude raises at two separate domains. A specific frequency increase was observed in the same domains and was accompanied by a global, unspecific raise. Amplitude and frequency increments were lower at sites initially more active, consistent with local compensatory processes. Transient preincubation with a specific Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitor either blocked or occluded amplitude and frequency upregulation in different synapse populations. Results are consistent with the concurrent development of different known CaMKII-dependent HSP processes. Our observations support that deafferentation causes rapid and diverse compensations resembling classical slow forms of adaptation to inactivity. These results may contribute to understand fast-developing homeostatic or pathological events after brain injury.
Keywords: CA1 pyramidal neurons; CaMKII; deafferentation; homeostatic synaptic plasticity; rat brain slices.
Copyright © 2022 Vergara, Pino, Vera, Arancibia and Sanhueza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bacci A., Coco S., Pravettoni E., Schenk U., Armano S., Frassoni C., et al. (2001). Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin–vesicle-associated membrane protein 2. J. Neurosci. 21 6588–6596. 10.1523/JNEUROSCI.21-17-06588.2001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

