Acute Administration of Metformin Protects Against Neuronal Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury via Regulation of the AMPK/CREB/BDNF Pathway
- PMID: 35431946
- PMCID: PMC9010658
- DOI: 10.3389/fphar.2022.832611
Acute Administration of Metformin Protects Against Neuronal Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury via Regulation of the AMPK/CREB/BDNF Pathway
Erratum in
-
Corrigendum: Acute Administration of Metformin Protects Against Neuronal Apoptosis Induced by Cerebral Ischemia-Reperfusion Injury via Regulation of the AMPK/CREB/BDNF Pathway.Front Pharmacol. 2022 Jun 2;13:929835. doi: 10.3389/fphar.2022.929835. eCollection 2022. Front Pharmacol. 2022. PMID: 35721130 Free PMC article.
Abstract
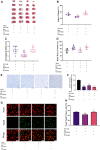
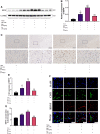
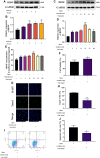
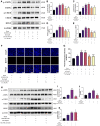
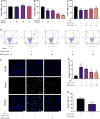
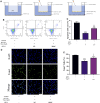
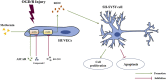
Metformin is a first-line anti-diabetic agent with a powerful hypoglycemic effect. Several studies have reported that metformin can improve the prognosis of stroke patients and that this effect is independent of its hypoglycemic effect; however, the specific mechanism remains unclear. In this research, we explored the effect and specific mechanism of metformin in cerebral ischemia-reperfusion (I/R) injury by constructing a transient middle cerebral artery occlusion model in vivo and a glucose and oxygen deprivation/reoxygenation (OGD/R) model in vitro. The results of the in vivo experiments showed that acute treatment with low-dose metformin (10 mg/kg) ameliorated cerebral edema, reduced the cerebral infarction volume, improved the neurological deficit score, and ameliorated neuronal apoptosis in the ischemic penumbra. Moreover, metformin up-regulated the brain-derived neurotrophic factor (BDNF) expression and increased phosphorylation levels of AMP-activated protein kinase (AMPK) and cAMP-response element binding protein (CREB) in the ischemia penumbra. Nevertheless, the above-mentioned effects of metformin were reversed by Compound C. The results of the in vitro experiments showed that low metformin concentrations (20 μM) could reduce apoptosis of human umbilical vein endothelial cells (HUVECs) under OGD/R conditions and promote cell proliferation. Moreover, metformin could further promote BDNF expression and release in HUVECs under OGD/R conditions via the AMPK/CREB pathway. The Transwell chamber assay showed that HUVECs treated with metformin could reduce apoptosis of SH-SY5Y cells under OGD/R conditions and this effect could be partially reversed by transfection of BDNF siRNA in HUVECs. In summary, our results suggest that metformin upregulates the level of BDNF in the cerebral ischemic penumbra via the AMPK/CREB pathway, thereby playing a protective effect in cerebral I/R injury.
Keywords: AMP-activated protein kinase; brain-derived neurotrophic factor; cerebral ischemia-reperfusion injury; metformin; neuronal apoptosis.
Copyright © 2022 Liu, Li, Liu, Li, Wu, Jiang, Wang, Chang, Jiang, Luo, Zhu, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. (2015). Pre-treatment with Metformin Activates Nrf2 Antioxidant Pathways and Inhibits Inflammatory Responses through Induction of AMPK after Transient Global Cerebral Ischemia. Metab. Brain Dis. 30 (3), 747–754. 10.1007/s11011-014-9632-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources

