Endothelial Transcytosis in Acute Lung Injury: Emerging Mechanisms and Therapeutic Approaches
- PMID: 35431977
- PMCID: PMC9008570
- DOI: 10.3389/fphys.2022.828093
Endothelial Transcytosis in Acute Lung Injury: Emerging Mechanisms and Therapeutic Approaches
Abstract
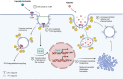
Acute Lung Injury (ALI) is characterized by widespread inflammation which in its severe form, Acute Respiratory Distress Syndrome (ARDS), leads to compromise in respiration causing hypoxemia and death in a substantial number of affected individuals. Loss of endothelial barrier integrity, pneumocyte necrosis, and circulating leukocyte recruitment into the injured lung are recognized mechanisms that contribute to the progression of ALI/ARDS. Additionally, damage to the pulmonary microvasculature by Gram-negative and positive bacteria or viruses (e.g., Escherichia coli, SARS-Cov-2) leads to increased protein and fluid permeability and interstitial edema, further impairing lung function. While most of the vascular leakage is attributed to loss of inter-endothelial junctional integrity, studies in animal models suggest that transendothelial transport of protein through caveolar vesicles, known as transcytosis, occurs in the early phase of ALI/ARDS. Here, we discuss the role of transcytosis in healthy and injured endothelium and highlight recent studies that have contributed to our understanding of the process during ALI/ARDS. We also cover potential approaches that utilize caveolar transport to deliver therapeutics to the lungs which may prevent further injury or improve recovery.
Keywords: PV-1; Src signaling; acute lung injury; caveolae (caveolin-1); endocytosis; endothelial permeability.
Copyright © 2022 Jones and Minshall.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bae M., Rusu L., Castellon M., Stojanovic-Terpo A., Onyuksel H., Du X., et al. (2019). Novel endothelial cell targeted peptide nanoformulation for inhibiting von Willebrand factor secretion to reduce thrombotic complications in sepsis. FASEB J. 33 680.11–680.11.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

