Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events
- PMID: 35432346
- PMCID: PMC9005797
- DOI: 10.3389/fimmu.2022.804597
Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events
Abstract
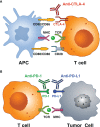
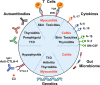
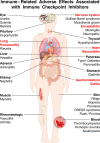
Immune checkpoint inhibitors (ICIs) block inhibitory molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or its ligand, programmed cell death protein ligand 1 (PD-L1) and enhance antitumor T-cell activity. ICIs provide clinical benefits in a percentage of patients with advanced cancers, but they are usually associated with a remarkable spectrum of immune-related adverse events (irAEs) (e.g., rash, colitis, hepatitis, pneumonitis, endocrine, cardiac and musculoskeletal dysfunctions). Particularly patients on combination therapy (e.g., anti-CTLA-4 plus anti-PD-1/PD-L1) experience some form of irAEs. Different mechanisms have been postulated to explain these adverse events. Host factors such as genotype, gut microbiome and pre-existing autoimmune disorders may affect the risk of adverse events. Fatal ICI-related irAEs are due to myocarditis, colitis or pneumonitis. irAEs usually occur within the first months after ICI initiation but can develop as early as after the first dose to years after ICI initiation. Most irAEs resolve pharmacologically, but some appear to be persistent. Glucocorticoids represent the mainstay of management of irAEs, but other immunosuppressive drugs can be used to mitigate refractory irAEs. In the absence of specific trials, several guidelines, based on data from retrospective studies and expert consensus, have been published to guide the management of ICI-related irAEs.
Keywords: PD-L1; cancer; cytotoxic T lymphocyte-associated protein (CTLA-4); immune checkpoint inhibitor (ICI); immune-related adverse event (irAE); immunotherapy; programmed cell death protein -1 (PD-1).
Copyright © 2022 Poto, Troiani, Criscuolo, Marone, Ciardiello, Tocchetti and Varricchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov (2018) 8:1069–86. doi: 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

