An Update of Cutaneous Melanoma Patients Treated in Adjuvancy With the Allogeneic Melanoma Vaccine VACCIMEL and Presentation of a Selected Case Report With In-Transit Metastases
- PMID: 35432383
- PMCID: PMC9011367
- DOI: 10.3389/fimmu.2022.842555
An Update of Cutaneous Melanoma Patients Treated in Adjuvancy With the Allogeneic Melanoma Vaccine VACCIMEL and Presentation of a Selected Case Report With In-Transit Metastases
Abstract
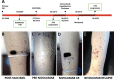
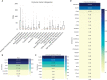
The CSF-470 vaccine (VACCIMEL) plus BCG and GM-CSF as adjuvants has been assayed in cutaneous melanoma patients. In the adjuvant randomized Phase II study CASVAC-0401, vaccinated patients had longer distant metastasis-free survival (DMFS) than those treated with IFNα2b. Five years after locking the data, an actualization was performed. The benefit in DMFS was maintained in the vaccinated group versus the IFNα2b-treated group (p = 0.035), with a median DMFS of 96 months for VACCIMEL and 13 months for IFNα2b. The favorable risk-benefit ratio was maintained. DMFS was also analyzed as a single cohort in all the IIB, IIC, and III patients (n = 30) who had been treated with VACCIMEL. The median DMFS was 169 months, and at 48 months follow-up, it was 71.4%, which was not statistically different from DMFS of previously published results obtained in adjuvancy with ipilimumab, pembrolizumab, nivolumab, or dabrafenib/trametinib. The possible toxicity of combining VACCIMEL with anti-immune checkpoint inhibitors (ICKi) was analyzed, especially since VACCIMEL was co-adjuvated with BCG in every vaccination. A patient with in-transit metastases was studied to produce a proof of concept. During treatment with VACCIMEL, the patient developed T-cell clones reactive towards tumor-associated antigens. Three years after ending the VACCIMEL study, the patient progressed and was treated with ICKi. During ICKi treatment, the patient did not reveal any toxicity due to previous BCG treatment. When she recurred after a 4-year treatment with nivolumab, a biopsy was obtained and immunohistochemistry and RNA-seq were performed. The tumor maintained expression of tumor-associated antigens and HLA-I and immune infiltration, with immunoreactive and immunosuppressive features. VACCIMEL plus BCG and GM-CSF is an effective treatment in adjuvancy for stages IIB, IIC, and III cutaneous melanoma patients, and it is compatible with subsequent treatments with ICKi.
Keywords: immune checkpoint inhibitor (ICKi); Bacille Calmette-Guérin (BCG); VACCIMEL; adjuvancy; cutaneous melanoma (CM); therapeutic vaccine.
Copyright © 2022 Mordoh, Aris, Carri, Bravo, Podaza, Pardo, Cueto, Barrio and Mordoh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. . Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB-C and Stage IV Melanoma (CheckMate 238): 4-Year Results From a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2020) 21:1465–77. doi: 10.1016/S1470-2045(20)30494-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

