The Function and Photoregulatory Mechanisms of Cryptochromes From Moso Bamboo (Phyllostachys edulis)
- PMID: 35432389
- PMCID: PMC9006058
- DOI: 10.3389/fpls.2022.866057
The Function and Photoregulatory Mechanisms of Cryptochromes From Moso Bamboo (Phyllostachys edulis)
Abstract
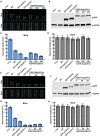
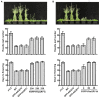
Light is one of the most important environmental factors affecting growth and geographic distribution of forestry plants. Moso bamboo is the largest temperate bamboo on earth and an important non-woody forestry species that serves not only important functions in the economy of rural areas but also carbon sequestration in the world. Due to its decades-long reproductive timing, the germplasm of moso bamboo cannot be readily improved by conventional breeding methods, arguing for a greater need to study the gene function and regulatory mechanisms of this species. We systematically studied the photoregulatory mechanisms of the moso bamboo (Phyllostachys edulis) cryptochrome 1, PheCRY1. Our results show that, similar to its Arabidopsis counterpart, the bamboo PheCRY1s are functionally restricted to the blue light inhibition of cell elongation without an apparent activity in promoting floral initiation. We demonstrate that PheCRY1s undergo light-dependent oligomerization that is inhibited by PheBIC1s, and light-dependent phosphorylation that is catalyzed by PhePPKs. We hypothesize that light-induced phosphorylation of PheCRY1s facilitate their degradation, which control availability of the PheCRY1 proteins and photosensitivity of bamboo plants. Our results demonstrate the evolutionary conservation of not only the function but also photoregulatory mechanism of PheCRY1 in this monocot forestry species.
Keywords: PheBIC; PhePPK; Phyllostachys edulis; cryptochrome; light signaling.
Copyright © 2022 Chen, Li, Liu, Chen, Zhang, Zhu, Kohnen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

