(Fluoro)alkylation of alkenes promoted by photolysis of alkylzirconocenes
- PMID: 35432852
- PMCID: PMC8943901
- DOI: 10.1039/d1sc07061d
(Fluoro)alkylation of alkenes promoted by photolysis of alkylzirconocenes
Abstract
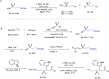
Difluoroalkylated compounds have important applications in pharmaceutical, agrochemical, and materials science. However, efficient methods to construct the alkylCF2-alkyl bond are very limited, and the site-selective introduction of a difluoromethylene (CF2) group into an aliphatic chain at the desired position remains challenging. Here, we report an unprecedented example of alkylzirconocene promoted difluoroalkylation of alkyl- and silyl-alkenes with a variety of unactivated difluoroalkyl iodides and bromides under the irradiation of visible light without a catalyst. The resulting difluoroalkylated compounds can serve as versatile synthons in organic synthesis. The reaction can also be applied to activated difluoroalkyl, trifluoromethyl, perfluoroalkyl, monofluoroalkyl, and nonfluorinated alkyl halides, providing a general method to controllably access fluorinated compounds. Preliminary mechanistic studies reveal that a single electron transfer (SET) pathway induced by a Zr(iii) species is involved in the reaction, in which the Zr(iii) species is generated by the photolysis of alkylzirconocene with blue light.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides.Acc Chem Res. 2018 Sep 18;51(9):2264-2278. doi: 10.1021/acs.accounts.8b00230. Epub 2018 Aug 22. Acc Chem Res. 2018. PMID: 30132322
-
Palladium-Catalyzed Difluoroalkylation of Isocyanides: Access to Difluoroalkylated Phenanthridine Derivatives.Org Lett. 2015 Nov 6;17(21):5384-7. doi: 10.1021/acs.orglett.5b02739. Epub 2015 Oct 26. Org Lett. 2015. PMID: 26502264
-
Visible-light induced three-component alkynyl-difluoroalkylation of unactivated alkenes.Chem Commun (Camb). 2018 Jul 12;54(57):7924-7927. doi: 10.1039/c8cc03105c. Chem Commun (Camb). 2018. PMID: 29951648
-
Nickel and cobalt-catalyzed coupling of alkyl halides with alkenes via heck reactions and radical conjugate addition.Mini Rev Med Chem. 2013 May 1;13(6):802-13. doi: 10.2174/1389557511313060003. Mini Rev Med Chem. 2013. PMID: 23544460 Review.
-
C-Difluoromethylene-containing, C-trifluoromethyl and C-perfluoroalkyl carbohydrates. Synthesis by carbohydrate transformation or building block methods.Carbohydr Res. 2000 Jul 10;327(1-2):119-46. doi: 10.1016/s0008-6215(00)00034-3. Carbohydr Res. 2000. PMID: 10968679 Review.
Cited by
-
Dialkylation of CF2 unit enabled by cobalt electron-shuttle catalysis.Nat Commun. 2024 Sep 10;15(1):7924. doi: 10.1038/s41467-024-51532-1. Nat Commun. 2024. PMID: 39256384 Free PMC article.
-
Catalytic Hydrodifluoroalkylation of Unactivated Olefins.Org Lett. 2022 Jul 22;24(28):5109-5114. doi: 10.1021/acs.orglett.2c01941. Epub 2022 Jul 10. Org Lett. 2022. PMID: 35815401 Free PMC article.
-
Programmable synthesis of difluorinated hydrocarbons from alkenes through a photocatalytic linchpin strategy.Chem Sci. 2023 Oct 11;14(41):11546-11553. doi: 10.1039/d3sc03951j. eCollection 2023 Oct 25. Chem Sci. 2023. PMID: 37886092 Free PMC article.
-
Asymmetric Synthesis of α-Chloroamides via Photoenzymatic Hydroalkylation of Olefins.J Am Chem Soc. 2024 Mar 20;146(11):7191-7197. doi: 10.1021/jacs.4c00927. Epub 2024 Mar 5. J Am Chem Soc. 2024. PMID: 38442365 Free PMC article.
References
-
- Hagmann W. K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. - DOI - PubMed
- Wang J. Sánchez-Roselló M. Aceña J. L. del Pozo C. Sorochinsky A. E. Fustero S. Soloshonok V. A. Liu H. Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. - DOI - PubMed
- Zhou Y. Wang J. Gu Z. Wang S. Zhu W. Aceña J. L. Soloshonok V. A. Izawa K. Liu H. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. - DOI - PubMed
- Inoue M. Sumii Y. Shibata N. ACS Omega. 2020;5:10633–10640. doi: 10.1021/acsomega.0c00830. - DOI - PMC - PubMed
- Ogawa Y. Tokunaga E. Kobayashi O. Hirai K. Shibata N. iScience. 2020;23:101467. doi: 10.1016/j.isci.2020.101467. - DOI - PMC - PubMed
-
- O'Hagan D. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. - DOI - PubMed
- Meanwell N. A. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. - DOI - PubMed
- Wang Y. Callejo R. Slawin A. M. Z. O'Hagan D. Beilstein J. Org. Chem. 2014;10:18–25. doi: 10.3762/bjoc.10.4. - DOI - PMC - PubMed
-
- Bégué J.-P. Bonnet-Delpon D. J. Fluorine Chem. 2006;127:992–1012. doi: 10.1016/j.jfluchem.2006.05.006. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

