Chemo- and regio-divergent access to fluorinated 1-alkyl and 1-acyl triazenes from alkynyl triazenes
- PMID: 35432853
- PMCID: PMC8943902
- DOI: 10.1039/d2sc00294a
Chemo- and regio-divergent access to fluorinated 1-alkyl and 1-acyl triazenes from alkynyl triazenes
Abstract
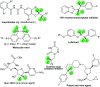
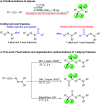
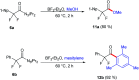
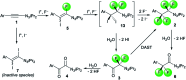
The 1,1,2,2-tetrafluoroethylene unit is prevalent in bioactive molecules and functional materials. Despite being in principle a straightforward strategy to access this motif, the direct tetrafluorination of alkynes involves very hazardous or inconvenient reagents. Therefore, safer and convenient alternatives are sought after. We developed a mild and operationally simple perfluorination method converting 1-alkynyl triazenes into 1,1,2,2-tetrafluoro alkyl triazenes, employing cheap and readily accessible reagents. Moreover, a judicious tuning of the reaction conditions enables access to α-difluoro triazenyl ketones. Complementary, electrophilic fluorination of alkynyl triazenes gives rise to the regioisomeric α-difluoro acyl triazenes. These three chemo- and regio-divergent protocols enable access to elusive fluorinated 1-alkyl and 1-acyl triazenes, thus expanding the chemical space for these unusual entities. Furthermore, several reaction intermediates and side products revealed insights on the reaction pathways that may be useful for further fluorination chemistry of alkynes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Vinyl and Alkynyl Triazenes: Synthesis, Reactivity, and Applications.Angew Chem Int Ed Engl. 2021 Mar 22;60(13):6879-6889. doi: 10.1002/anie.202011031. Epub 2020 Nov 3. Angew Chem Int Ed Engl. 2021. PMID: 33142011 Review.
-
Alkynyl triazenes enable divergent syntheses of 2-pyrones.Chem Sci. 2021 Jun 4;12(26):9140-9145. doi: 10.1039/d1sc02583j. eCollection 2021 Jul 7. Chem Sci. 2021. PMID: 34276943 Free PMC article.
-
Light-Induced Metal-Free Generation of Cyanocarbenes from Alkynyl Triazenes for the Synthesis of Nitrile Derivatives.Angew Chem Int Ed Engl. 2023 Oct 16;62(42):e202309274. doi: 10.1002/anie.202309274. Epub 2023 Sep 8. Angew Chem Int Ed Engl. 2023. PMID: 37515774
-
Divergent Synthesis of Densely Substituted Arenes and Pyridines via Cyclotrimerization Reactions of Alkynyl Triazenes.J Am Chem Soc. 2019 Jul 3;141(26):10372-10383. doi: 10.1021/jacs.9b04111. Epub 2019 Jun 24. J Am Chem Soc. 2019. PMID: 31244170
-
Gold-catalyzed fluorination of alkynes/allenes: mechanistic explanations and reaction scope.Org Biomol Chem. 2024 Dec 18;23(1):11-35. doi: 10.1039/d4ob01579g. Org Biomol Chem. 2024. PMID: 39513472 Review.
Cited by
-
Expanding the chemical space of enol silyl ethers: catalytic dicarbofunctionalization enabled by iron catalysis.Chem Sci. 2023 Oct 20;14(45):13007-13013. doi: 10.1039/d3sc04549h. eCollection 2023 Nov 22. Chem Sci. 2023. PMID: 38023494 Free PMC article.
-
Nitrous oxide as diazo transfer reagent.Chem Sci. 2024 Aug 14;15(34):13605-17. doi: 10.1039/d4sc04530k. Online ahead of print. Chem Sci. 2024. PMID: 39156938 Free PMC article. Review.
References
-
- Ni C. Hu J. Chem. Soc. Rev. 2016;45:5441–5454. doi: 10.1039/C6CS00351F. - DOI - PubMed
- Hagmann W. K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. - DOI - PubMed
- O'Hagan D. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. - DOI - PubMed
- Purser S. Moore P. R. Swallow S. Gouverneur V. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. - DOI - PubMed
-
- Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, ed. V. Gouverneur and K. Müller, Imperial College Press, London, UK, 2012, vol. 6, pp. 1–558
- Fluorine in Medicinal Chemistry and Chemical Biology, ed. I. Ojima, Wiley-Blackwell, Chichester, UK, 2009, pp. 1–640
- Müller K. Faeh C. Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. - DOI - PubMed
- Jeschke P. ChemBioChem. 2004;5:570–589. doi: 10.1002/cbic.200300833. - DOI - PubMed
- Harper D. B. O'Hagan D. Nat. Prod. Rep. 1994;11:123–133. doi: 10.1039/NP9941100123. - DOI - PubMed
-
- Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, ed. P. Kirsch, Wiley-VCH, Weinheim, 2006, pp. 1–320
- Fluorine in Organic Chemistry, ed. R. D. Chambers, Blackwell Publishing Ltd, Oxford, 2004, pp. 1–406
-
- Fujiwara T. O'Hagan D. J. Fluorine Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. - DOI
- Giornal F. Pazenok S. Rodefeld L. Lui N. Vors J. P. Leroux F. R. J. Fluorine Chem. 2013;152:2–11. doi: 10.1016/j.jfluchem.2012.11.008. - DOI
- Boyer J. Arnoult E. Médebielle M. Guillemont J. Unge J. Jochmans D. J. Med. Chem. 2011;54:7974–7985. doi: 10.1021/jm200766b. - DOI - PubMed
- Jeschke P. Pest Manage. Sci. 2010;66:10–27. doi: 10.1002/ps.1829. - DOI - PubMed
- Christy M. E. Colton C. D. Mackay M. Staas W. H. Wong J. B. Engelhardt E. L. Torchiana M. L. Stone C. A. J. Med. Chem. 1977;20:421–430. doi: 10.1021/jm00213a021. - DOI - PubMed
-
- Jia R. Wang X. Hu J. Tetrahedron Lett. 2021;75:153182. doi: 10.1016/j.tetlet.2021.153182. - DOI
- Miele M. Pace V. Aust. J. Chem. 2021;74:623–625. doi: 10.1071/CH21045. - DOI
- Rong J. Ni C. Hu J. Asian J. Org. Chem. 2017;6:139–152. doi: 10.1002/ajoc.201600509. - DOI
- Ni C. Hu J. Chem. Soc. Rev. 2016;45:5441–5454. doi: 10.1039/C6CS00351F. - DOI - PubMed
- Belhomme M.-C. Besset T. Poisson T. Pannecouke X. Chem.–Eur. J. 2015;21:12836–12865. doi: 10.1002/chem.201501475. - DOI - PubMed
- Liang T. Neumann C. N. Ritter T. Angew. Chem., Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. - DOI - PubMed
LinkOut - more resources
Full Text Sources

