Chemo- and regio-divergent access to fluorinated 1-alkyl and 1-acyl triazenes from alkynyl triazenes
- PMID: 35432853
- PMCID: PMC8943902
- DOI: 10.1039/d2sc00294a
Chemo- and regio-divergent access to fluorinated 1-alkyl and 1-acyl triazenes from alkynyl triazenes
Abstract
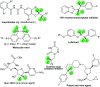
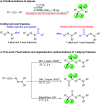
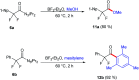
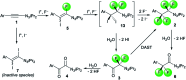
The 1,1,2,2-tetrafluoroethylene unit is prevalent in bioactive molecules and functional materials. Despite being in principle a straightforward strategy to access this motif, the direct tetrafluorination of alkynes involves very hazardous or inconvenient reagents. Therefore, safer and convenient alternatives are sought after. We developed a mild and operationally simple perfluorination method converting 1-alkynyl triazenes into 1,1,2,2-tetrafluoro alkyl triazenes, employing cheap and readily accessible reagents. Moreover, a judicious tuning of the reaction conditions enables access to α-difluoro triazenyl ketones. Complementary, electrophilic fluorination of alkynyl triazenes gives rise to the regioisomeric α-difluoro acyl triazenes. These three chemo- and regio-divergent protocols enable access to elusive fluorinated 1-alkyl and 1-acyl triazenes, thus expanding the chemical space for these unusual entities. Furthermore, several reaction intermediates and side products revealed insights on the reaction pathways that may be useful for further fluorination chemistry of alkynes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- Ni C. Hu J. Chem. Soc. Rev. 2016;45:5441–5454. doi: 10.1039/C6CS00351F. - DOI - PubMed
- Hagmann W. K. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. - DOI - PubMed
- O'Hagan D. Chem. Soc. Rev. 2008;37:308–319. doi: 10.1039/B711844A. - DOI - PubMed
- Purser S. Moore P. R. Swallow S. Gouverneur V. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. - DOI - PubMed
-
- Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, ed. V. Gouverneur and K. Müller, Imperial College Press, London, UK, 2012, vol. 6, pp. 1–558
- Fluorine in Medicinal Chemistry and Chemical Biology, ed. I. Ojima, Wiley-Blackwell, Chichester, UK, 2009, pp. 1–640
- Müller K. Faeh C. Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. - DOI - PubMed
- Jeschke P. ChemBioChem. 2004;5:570–589. doi: 10.1002/cbic.200300833. - DOI - PubMed
- Harper D. B. O'Hagan D. Nat. Prod. Rep. 1994;11:123–133. doi: 10.1039/NP9941100123. - DOI - PubMed
-
- Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, ed. P. Kirsch, Wiley-VCH, Weinheim, 2006, pp. 1–320
- Fluorine in Organic Chemistry, ed. R. D. Chambers, Blackwell Publishing Ltd, Oxford, 2004, pp. 1–406
-
- Fujiwara T. O'Hagan D. J. Fluorine Chem. 2014;167:16–29. doi: 10.1016/j.jfluchem.2014.06.014. - DOI
- Giornal F. Pazenok S. Rodefeld L. Lui N. Vors J. P. Leroux F. R. J. Fluorine Chem. 2013;152:2–11. doi: 10.1016/j.jfluchem.2012.11.008. - DOI
- Boyer J. Arnoult E. Médebielle M. Guillemont J. Unge J. Jochmans D. J. Med. Chem. 2011;54:7974–7985. doi: 10.1021/jm200766b. - DOI - PubMed
- Jeschke P. Pest Manage. Sci. 2010;66:10–27. doi: 10.1002/ps.1829. - DOI - PubMed
- Christy M. E. Colton C. D. Mackay M. Staas W. H. Wong J. B. Engelhardt E. L. Torchiana M. L. Stone C. A. J. Med. Chem. 1977;20:421–430. doi: 10.1021/jm00213a021. - DOI - PubMed
-
- Jia R. Wang X. Hu J. Tetrahedron Lett. 2021;75:153182. doi: 10.1016/j.tetlet.2021.153182. - DOI
- Miele M. Pace V. Aust. J. Chem. 2021;74:623–625. doi: 10.1071/CH21045. - DOI
- Rong J. Ni C. Hu J. Asian J. Org. Chem. 2017;6:139–152. doi: 10.1002/ajoc.201600509. - DOI
- Ni C. Hu J. Chem. Soc. Rev. 2016;45:5441–5454. doi: 10.1039/C6CS00351F. - DOI - PubMed
- Belhomme M.-C. Besset T. Poisson T. Pannecouke X. Chem.–Eur. J. 2015;21:12836–12865. doi: 10.1002/chem.201501475. - DOI - PubMed
- Liang T. Neumann C. N. Ritter T. Angew. Chem., Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. - DOI - PubMed
LinkOut - more resources
Full Text Sources

