Sterically demanding macrocyclic Eu(iii) complexes for selective recognition of phosphate and real-time monitoring of enzymatically generated adenosine monophosphate
- PMID: 35432862
- PMCID: PMC8943852
- DOI: 10.1039/d1sc05377a
Sterically demanding macrocyclic Eu(iii) complexes for selective recognition of phosphate and real-time monitoring of enzymatically generated adenosine monophosphate
Abstract
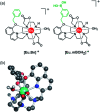
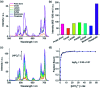
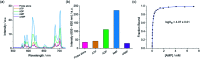
The design of molecular receptors that bind and sense anions in biologically relevant aqueous solutions is a key challenge in supramolecular chemistry. The recognition of inorganic phosphate is particularly challenging because of its high hydration energy and pH dependent speciation. Adenosine monophosphate (AMP) represents a valuable but elusive target for supramolecular detection because of its structural similarity to the more negatively charged anions, ATP and ADP. We report two new macrocyclic Eu(iii) receptors capable of selectively sensing inorganic phosphate and AMP in water. The receptors contain a sterically demanding 8-(benzyloxy)quinoline pendant arm that coordinates to the metal centre, creating a binding pocket suitable for phosphate and AMP, whilst excluding potentially interfering chelating anions, in particular ATP, bicarbonate and lactate. The sensing selectivity of our Eu(iii) receptors follows the order AMP > ADP > ATP, which represents a reversal of the order of selectivity observed for most reported nucleoside phosphate receptors. We have exploited the unique host-guest induced changes in emission intensity and lifetime for the detection of inorganic phosphate in human serum samples, and for monitoring the enzymatic production of AMP in real-time.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

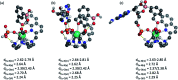


Similar articles
-
Lanthanide(III) complexes of bis-semicarbazone and bis-imine-substituted phenanthroline ligands: solid-state structures, photophysical properties, and anion sensing.Chemistry. 2012 Dec 21;18(52):16784-92. doi: 10.1002/chem.201201705. Epub 2012 Nov 13. Chemistry. 2012. PMID: 23150237
-
Exploring the binding ability of polyammonium hosts for anionic substrates: selective size-dependent recognition of different phosphate anions by bis-macrocyclic receptors.Inorg Chem. 2011 Aug 1;50(15):7202-16. doi: 10.1021/ic2007815. Epub 2011 Jun 28. Inorg Chem. 2011. PMID: 21710997
-
Chemosensors for pyrophosphate.Acc Chem Res. 2009 Jan 20;42(1):23-31. doi: 10.1021/ar800003f. Acc Chem Res. 2009. PMID: 18798656
-
Anion Receptors for the Discrimination of ATP and ADP in Biological Media.Chempluschem. 2021 Jan;86(1):59-70. doi: 10.1002/cplu.202000567. Epub 2020 Oct 15. Chempluschem. 2021. PMID: 33058508 Review.
-
Design and applications of metal-based molecular receptors and probes for inorganic phosphate.Chem Soc Rev. 2020 Feb 24;49(4):1090-1108. doi: 10.1039/c9cs00543a. Chem Soc Rev. 2020. PMID: 32016270 Review.
Cited by
-
Elucidating Polyphosphate Anion Binding to Lanthanide Complexes Using EXAFS and Pulsed EPR Spectroscopy.Inorg Chem. 2024 Oct 28;63(43):20726-20736. doi: 10.1021/acs.inorgchem.4c03399. Epub 2024 Oct 16. Inorg Chem. 2024. PMID: 39412769 Free PMC article.
-
Synthetic approaches to metal-coordination-directed macrocyclic complexes.Front Chem. 2022 Nov 24;10:1078432. doi: 10.3389/fchem.2022.1078432. eCollection 2022. Front Chem. 2022. PMID: 36505734 Free PMC article. Review.
-
Luminescent Lanthanide Probes for Inorganic and Organic Phosphates.Chem Asian J. 2022 Aug 15;17(16):e202200495. doi: 10.1002/asia.202200495. Epub 2022 Jul 5. Chem Asian J. 2022. PMID: 35750633 Free PMC article. Review.
-
Luminescent lanthanide probes for cations and anions: Promises, compromises, and caveats.Curr Opin Chem Biol. 2023 Oct;76:102374. doi: 10.1016/j.cbpa.2023.102374. Epub 2023 Jul 28. Curr Opin Chem Biol. 2023. PMID: 37517109 Free PMC article. Review.
-
Anion binding to a cationic europium(III) probe enables the first real-time assay of heparan sulfotransferase activity.Org Biomol Chem. 2022 Jan 19;20(3):596-605. doi: 10.1039/d1ob02071d. Org Biomol Chem. 2022. PMID: 34951618 Free PMC article.
References
LinkOut - more resources
Full Text Sources

