Catalysis with cycloruthenated complexes
- PMID: 35432864
- PMCID: PMC8943884
- DOI: 10.1039/d1sc06355c
Catalysis with cycloruthenated complexes
Abstract
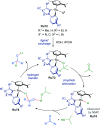
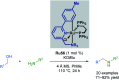
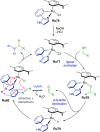
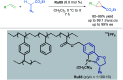
Cycloruthenated complexes have been studied extensively over the last few decades. Many accounts of their synthesis, characterisation, and catalytic activity in a wide variety of transformations have been reported to date. Compared with their non-cyclometallated analogues, cycloruthenated complexes may display enhanced catalytic activities in known transformations or possess entirely new reactivity. In other instances, these complexes can be chiral, and capable of catalysing stereoselective reactions. In this review, we aim to highlight the catalytic applications of cycloruthenated complexes in organic synthesis, emphasising the recent advancements in this field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

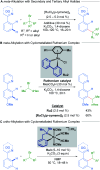
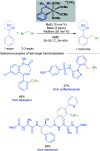
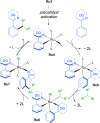






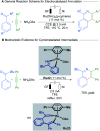
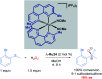
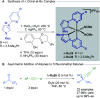

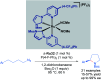


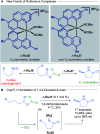
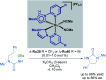
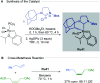


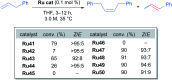
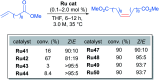





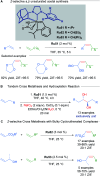
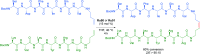
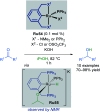

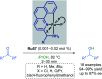
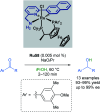
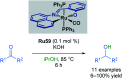
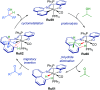

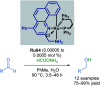






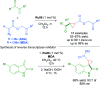







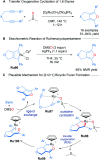
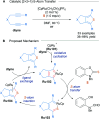

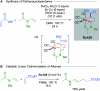
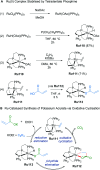
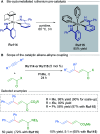





References
-
- Hegedus L. S., in Comprehensive Organometallic Chemistry II, ed. E. W. Abel, F. G. A. Stone and G. Wilkinson, Elsevier, Amsterdam, 1995, vol. 12
- Hagen J., in Industrial Catalysis, ed. J. Hagen, Wiley-VCH, Weinheim, 2015, ch. 2
-
- Herrmann W. A. Brossmer C. Reisinger C.-P. Riermeier T. H. Öfele K. Beller M. Chem.–Eur. J. 1997;3:1357–1364. doi: 10.1002/chem.19970030823. - DOI
- Martin R. Buchwald S. L. Acc. Chem. Res. 2008;41:1461–1473. doi: 10.1021/ar800036s. - DOI - PMC - PubMed
- Surry D. S. Buchwald S. L. Angew. Chem., Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. - DOI - PMC - PubMed
-
- Engelhard Industrial Bullion (EIB) Prices (USD per Troy Ounce),https://apps.catalysts.basf.com/apps/eibprices/mp/, accessed 29/07/2021, ruthenium = 750.00, platinum = 1051.00, palladium = 2626.00, rhodium = 18400.00
Publication types
LinkOut - more resources
Full Text Sources

