The convergent total synthesis and antibacterial profile of the natural product streptothricin F
- PMID: 35432870
- PMCID: PMC8943883
- DOI: 10.1039/d1sc06445b
The convergent total synthesis and antibacterial profile of the natural product streptothricin F
Abstract
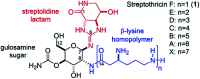
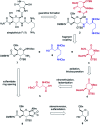
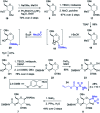
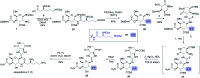
A convergent, diversity-enabling total synthesis of the natural product streptothricin F has been achieved. Herein, we describe the potent antimicrobial activity of streptothricin F and highlight the importance of a total synthesis that allows for the installation of practical divergent steps for medicinal chemistry exploits. Key features of our synthesis include a Burgess reagent-mediated 1,2-anti-diamine installation, diastereoselective azidation of a lactam enolate, and a mercury(ii) chloride-mediated desulfurization-guanidination. The development of this chemistry enables the synthesis and structure-activity studies of streptothricin F analogs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest. The HP D300 digital dispenser and TECAN M1000 used for MIC analysis in Table 1 were provided for our use by TECAN (Morrisville, NC). TECAN had no role in study design, data collection/interpretation, manuscript preparation, or decision to publish.
Figures

Similar articles
-
Isolation, structure elucidation and antibacterial activities of streptothricin acids.J Antibiot (Tokyo). 2009 May;62(5):233-7. doi: 10.1038/ja.2009.16. Epub 2009 Mar 20. J Antibiot (Tokyo). 2009. PMID: 19300469
-
Nourseothricin (streptothricin) inactivated by a plasmid pIE636 encoded acetyl transferase of Escherichia coli: location of the acetyl group.FEMS Microbiol Lett. 1993 Jul 1;110(3):331-4. doi: 10.1111/j.1574-6968.1993.tb06344.x. FEMS Microbiol Lett. 1993. PMID: 8394835
-
Action of streptothricin F on ribosomal functions.J Antibiot (Tokyo). 1980 Jun;33(6):636-41. doi: 10.7164/antibiotics.33.636. J Antibiot (Tokyo). 1980. PMID: 6252135
-
[Convergent Synthesis of Fused Ring Systems in Large Polycyclic Ethers].Yakugaku Zasshi. 2017;137(9):1095-1101. doi: 10.1248/yakushi.17-00116. Yakugaku Zasshi. 2017. PMID: 28867696 Review. Japanese.
-
[Progress in streptothricin antibiotics - A review].Wei Sheng Wu Xue Bao. 2016 Mar 4;56(3):363-72. Wei Sheng Wu Xue Bao. 2016. PMID: 27382780 Review. Chinese.
Cited by
-
Nourseothricin as a novel therapeutic agent against Neisseria gonorrhoeae: in vitro and in vivo evaluations using Galleria mellonella-a pilot study.Microbiol Spectr. 2025 Aug 5;13(8):e0127525. doi: 10.1128/spectrum.01275-25. Epub 2025 Jul 15. Microbiol Spectr. 2025. PMID: 40662736 Free PMC article.
-
Pharmacological and therapeutic effects of natural products on liver regeneration-a comprehensive research.Chin Med. 2025 May 6;20(1):57. doi: 10.1186/s13020-025-01108-y. Chin Med. 2025. PMID: 40329344 Free PMC article. Review.
-
Exploration of the Fusidic Acid Structure Activity Space for Antibiotic Activity.Molecules. 2025 Jan 21;30(3):465. doi: 10.3390/molecules30030465. Molecules. 2025. PMID: 39942570 Free PMC article.
-
History of the streptothricin antibiotics and evidence for the neglect of the streptothricin resistome.NPJ Antimicrob Resist. 2024 Feb 7;2(1):3. doi: 10.1038/s44259-023-00020-5. NPJ Antimicrob Resist. 2024. PMID: 39843956 Free PMC article. Review.
-
Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome.PLoS Biol. 2023 May 16;21(5):e3002091. doi: 10.1371/journal.pbio.3002091. eCollection 2023 May. PLoS Biol. 2023. PMID: 37192172 Free PMC article.
References
-
- Waksman S. A. Woodruff H. B. Streptothricin, a new selective bacteriostatic and bactericidal agent, particularly active against gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 1942;49(2):207–210. doi: 10.3181/00379727-49-13515. - DOI
-
- Lumb M. Chamberlain N. Cross T. Macey P. E. Spyvee J. Uprichard J. M. Wright R. D. An antibiotic complex (A4788) containing streptothricin and having activity against powdery mildews. J. Sci. Food Agric. 1962;13(6):343–352. doi: 10.1002/jsfa.2740130607. - DOI
-
- Johnson A. W. Westley J. W. The streptothricin group of antibiotics. Part I. the general structural pattern. J. Chem. Soc. 1962;1042:1642. doi: 10.1039/JR9620001642. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

