Distinctive features and challenges in catenane chemistry
- PMID: 35432874
- PMCID: PMC8943846
- DOI: 10.1039/d1sc05391d
Distinctive features and challenges in catenane chemistry
Abstract
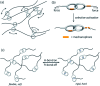
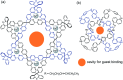
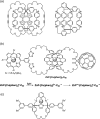
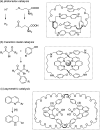
From being an aesthetic molecular object to a building block for the construction of molecular machines, catenanes and related mechanically interlocked molecules (MIMs) continue to attract immense interest in many research areas. Catenane chemistry is closely tied to that of rotaxanes and knots, and involves concepts like mechanical bonds, chemical topology and co-conformation that are unique to these molecules. Yet, because of their different topological structures and mechanical bond properties, there are some fundamental differences between the chemistry of catenanes and that of rotaxanes and knots although the boundary is sometimes blurred. Clearly distinguishing these differences, in aspects of bonding, structure, synthesis and properties, between catenanes and other MIMs is therefore of fundamental importance to understand their chemistry and explore the new opportunities from mechanical bonds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Catenane and Rotaxane Synthesis from Cucurbit[6]uril-Mediated Azide-Alkyne Cycloaddition.Chem Asian J. 2023 Sep 1;18(17):e202300290. doi: 10.1002/asia.202300290. Epub 2023 Jul 31. Chem Asian J. 2023. PMID: 37460745 Review.
-
Ground-state kinetics of bistable redox-active donor-acceptor mechanically interlocked molecules.Acc Chem Res. 2014 Feb 18;47(2):482-93. doi: 10.1021/ar400161z. Epub 2013 Dec 16. Acc Chem Res. 2014. PMID: 24341283
-
Lanthanide-Containing Rotaxanes, Catenanes, and Knots.Chempluschem. 2020 Apr;85(4):783-792. doi: 10.1002/cplu.202000135. Chempluschem. 2020. PMID: 32319722 Review.
-
Rotaxane Dendrimers: Alliance between Giants.Acc Chem Res. 2021 Nov 2;54(21):4091-4106. doi: 10.1021/acs.accounts.1c00507. Epub 2021 Oct 22. Acc Chem Res. 2021. PMID: 34676764
-
Synthesis of rotaxanes and catenanes using an imine clipping reaction.Org Biomol Chem. 2016 Nov 8;14(44):10331-10351. doi: 10.1039/c6ob01581f. Org Biomol Chem. 2016. PMID: 27714207 Review.
Cited by
-
Dynamic mechanostereochemical switching of a co-conformationally flexible [2]catenane controlled by specific ionic guests.Nat Commun. 2024 Mar 4;15(1):1952. doi: 10.1038/s41467-024-46099-w. Nat Commun. 2024. PMID: 38433258 Free PMC article.
-
Assembling a new generation of radiopharmaceuticals with supramolecular theranostics.Nat Rev Chem. 2024 Dec;8(12):893-914. doi: 10.1038/s41570-024-00657-4. Epub 2024 Oct 28. Nat Rev Chem. 2024. PMID: 39468298 Review.
-
Mechanical and Covalent Tailoring of Copper Catenanes for Selective Aqueous Nitrate-to-Ammonia Electrocatalysis.J Am Chem Soc. 2025 Apr 30;147(17):14316-14325. doi: 10.1021/jacs.4c18547. Epub 2025 Apr 22. J Am Chem Soc. 2025. PMID: 40260598 Free PMC article.
-
A Copper(I) Catenane Decorated Metal-Organic Layer as a Heterogenous Catalyst for Dehydrogenative Cross-Coupling.Chemistry. 2025 Jun 3;31(31):e202500866. doi: 10.1002/chem.202500866. Epub 2025 Apr 26. Chemistry. 2025. PMID: 40241498 Free PMC article.
-
Stimuli-Responsive Catenane-Based Catalysts.Angew Chem Int Ed Engl. 2023 Feb 1;62(6):e202216787. doi: 10.1002/anie.202216787. Epub 2022 Dec 28. Angew Chem Int Ed Engl. 2023. PMID: 36478644 Free PMC article.
References
-
- Bruns C. J. and Stoddart J. F., The Nature of Mechanical Bond: from Molecules to Machines, John Wiley & Sons, New Jersey, 2017
- Guo Q.-H. Jiao Y. Feng Y. Stoddart J. F. CCS Chem. 2021;3:1542. doi: 10.31635/ccschem.021.202100975. - DOI
- Sluysmans D. Stoddart J. F. Trends Chem. 2019;1:185. doi: 10.1016/j.trechm.2019.02.013. - DOI
- Dietrich-Buchecker C. O. Sauvage J. P. Chem. Rev. 1987;87:795. doi: 10.1021/cr00080a007. - DOI
-
- Fielden S. D. P. Leigh D. A. Woltering S. L. Angew. Chem., Int. Ed. 2017;56:11166. doi: 10.1002/anie.201702531. - DOI - PMC - PubMed
- Beves J. E. Blight B. A. Campbell C. J. Leigh D. A. McBurney R. T. Angew. Chem., Int. Ed. 2011;50:9260. doi: 10.1002/anie.201007963. - DOI - PubMed
- Huang S.-L. Hor T. S. A. Jin G.-X. Coord. Chem. Rev. 2017;333:1. doi: 10.1016/j.ccr.2016.11.009. - DOI
- Gao W.-X. Feng H.-J. Guo B.-B. Lu Y. Jin G.-X. Chem. Rev. 2020;120:6288. doi: 10.1021/acs.chemrev.0c00321. - DOI - PubMed
-
- Mingos D. M. P. Yau J. Menzer S. Williams D. J. Angew. Chem., Int. Ed. Engl. 1995;34:1894. doi: 10.1002/anie.199518941. - DOI
- McArdle C. P. Jennings M. C. Vittal J. J. Puddephatt R. J. Chem.–Eur. J. 2001;7:3572. doi: 10.1002/1521-3765(20010817)7:16<3572::AID-CHEM3572>3.0.CO;2-C. - DOI - PubMed
- Chui S. S.-Y. Chen R. Che C. M. Angew. Chem., Int. Ed. 2006;45:1621. doi: 10.1002/anie.200503431. - DOI - PubMed
- Xu G.-T. Wu L.-L. Chang X.-Y. Ang T. W. H. Wong W.-Y. Huang J.-S. Che C.-M. Angew. Chem., Int. Ed. 2019;58:16297. doi: 10.1002/anie.201909980. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

