Synergistic Brønsted/Lewis acid catalyzed aromatic alkylation with unactivated tertiary alcohols or di- tert-butylperoxide to synthesize quaternary carbon centers
- PMID: 35432882
- PMCID: PMC8943850
- DOI: 10.1039/d1sc06422c
Synergistic Brønsted/Lewis acid catalyzed aromatic alkylation with unactivated tertiary alcohols or di- tert-butylperoxide to synthesize quaternary carbon centers
Abstract
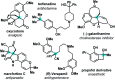
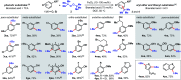
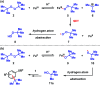
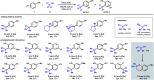
Dual Brønsted/Lewis acid catalysis involving environmentally benign, readily accessible protic acid and iron promotes site-selective tert-butylation of electron-rich arenes using di-tert-butylperoxide. This transformation inspired the development of a synergistic Brønsted/Lewis acid catalyzed aromatic alkylation that fills a gap in the Friedel-Crafts reaction literature by employing unactivated tertiary alcohols as alkylating agents, leading to new quaternary carbon centers. Corroborated by DFT calculations, the Lewis acid serves a role in enhancing the acidity of the Brønsted acid. The use of non-allylic, non-benzylic, and non-propargylic tertiary alcohols represents an underexplored area in Friedel-Crafts reactivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Two-component boronic acid catalysis for increased reactivity in challenging Friedel-Crafts alkylations with deactivated benzylic alcohols.Org Biomol Chem. 2019 Jun 18;17(24):6007-6014. doi: 10.1039/c9ob01043b. Org Biomol Chem. 2019. PMID: 31166346
-
Iron-catalyzed oxidative coupling of alkylamides with arenes through oxidation of alkylamides followed by Friedel-Crafts alkylation.J Org Chem. 2011 Jan 7;76(1):25-34. doi: 10.1021/jo102217m. Epub 2010 Dec 15. J Org Chem. 2011. PMID: 21158393
-
Selective benzylic and allylic alkylation of protic nucleophiles with sulfonamides through double Lewis acid catalyzed cleavage of sp3 carbon-nitrogen bonds.Chemistry. 2009;15(3):793-7. doi: 10.1002/chem.200801665. Chemistry. 2009. PMID: 19035590
-
Direct Substitution of Alcohols in Pure Water by Brønsted Acid Catalysis.Molecules. 2017 Apr 1;22(4):574. doi: 10.3390/molecules22040574. Molecules. 2017. PMID: 28368309 Free PMC article. Review.
-
Generation of C(sp3)-CAr bonds in the synthesis of triarylmethanes (TRAMs): comprehensive progress since 2009.Org Biomol Chem. 2025 Apr 9;23(15):3492-3519. doi: 10.1039/d4ob02069c. Org Biomol Chem. 2025. PMID: 40084579 Review.
Cited by
-
Semisynthesis of bersavine and berbamine derivatives that target the CaMKIIγ:cMyc axis for lymphoma therapy.Org Biomol Chem. 2025 May 7;23(18):4403-4408. doi: 10.1039/d5ob00310e. Org Biomol Chem. 2025. PMID: 40202443
-
A Fenton Approach to Aromatic Radical Cations and Diarylmethane Synthesis.J Org Chem. 2023 Nov 3;88(21):15060-15066. doi: 10.1021/acs.joc.3c01505. Epub 2023 Oct 17. J Org Chem. 2023. PMID: 37847050 Free PMC article.
-
Generalizing arene C-H alkylations by radical-radical cross-coupling.Nature. 2025 May;641(8061):112-121. doi: 10.1038/s41586-025-08887-2. Epub 2025 Mar 24. Nature. 2025. PMID: 40127680
References
-
- Méndez-Lucio O. Medina-Franco J. L. Drug Discovery Today. 2017;22:120–126. doi: 10.1016/j.drudis.2016.08.009. - DOI - PubMed
- Dombrowski A. W. Gesmundo N. J. Aguirre A. L. Sarris K. A. Young J. M. Bogdan A. R. Martin M. C. Gedeon S. Wang Y. ACS Med. Chem. Lett. 2020;11:597–604. doi: 10.1021/acsmedchemlett.0c00093. - DOI - PMC - PubMed
-
- Long R. Huang J. Gong J. Yang Z. Nat. Prod. Rep. 2015;32:1584–1601. doi: 10.1039/C5NP00046G. - DOI - PubMed
- Ling T. Rivas F. Tetrahedron. 2016;72:6729–6777. doi: 10.1016/j.tet.2016.09.002. - DOI
- Talele T. T. J. Med. Chem. 2020;63:13291–13315. doi: 10.1021/acs.jmedchem.0c00829. - DOI - PubMed
- Zhang C. Li F. Yu Y. Huang A. He P. Lei M. Wang J. Huang L. Liu Z. Liu J. Wei Y. J. Med. Chem. 2017;60:3618–3625. doi: 10.1021/acs.jmedchem.7b00253. - DOI - PubMed
-
-
For selected reviews, see:
- Douglas C. J. Overman L. E. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. - DOI - PMC - PubMed
- Quasdorf K. W. Overman L. E. Nature. 2014;516:181–191. doi: 10.1038/nature14007. - DOI - PMC - PubMed
- Das J. P. Marek I. Chem. Commun. 2011;47:4593–4623. doi: 10.1039/C0CC05222A. - DOI - PubMed
-

