Oxindole synthesis via polar-radical crossover of ketene-derived amide enolates in a formal [3 + 2] cycloaddition
- PMID: 35432887
- PMCID: PMC8966637
- DOI: 10.1039/d1sc07134c
Oxindole synthesis via polar-radical crossover of ketene-derived amide enolates in a formal [3 + 2] cycloaddition
Abstract
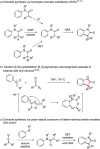
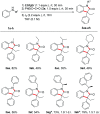
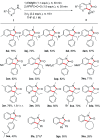
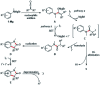
Herein we introduce a simple, efficient and transition-metal free method for the preparation of valuable and sterically hindered 3,3-disubstituted oxindoles via polar-radical crossover of ketene derived amide enolates. Various easily accessible N-alkyl and N-arylanilines are added to disubstituted ketenes and the resulting amide enolates undergo upon single electron transfer oxidation a homolytic aromatic substitution (HAS) to provide 3,3-disubstituted oxindoles in good to excellent yields. A variety of substituted anilines and a 3-amino pyridine engage in this oxidative formal [3 + 2] cycloaddition and cyclic ketenes provide spirooxindoles. Both substrates and reagents are readily available and tolerance to functional groups is broad.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
-
For selected reviews see:
- Dreifuss A. A. Bastos-Pereira A. L. Avila T. V. Da Soley B. Rivero A. J. Aguilar J. L. Acco A. J. Ethnopharmacology. 2010;130:127. doi: 10.1016/j.jep.2010.04.029. - DOI - PubMed
- Kaur M. Chadha N. Silakari O. Eur. J. Med. Chem. 2016;123:858. doi: 10.1016/j.ejmech.2016.08.011. - DOI - PubMed
- Khetmalis Y. M. Shivani M. Murugesan S. Chandra Sekhar K. V. G. Biomed. Pharmacother. 2021;141:111842. doi: 10.1016/j.biopha.2021.111842. - DOI - PubMed
-
-
-
For selected natural products see:
- Anderton N. Cockrum P. A. Colegate S. M. Edgar J. A. Flower K. Vit I. Willing R. I. Phytochemistry. 1998;48:437. doi: 10.1016/S0031-9422(97)00946-1. - DOI
- Jossang A. Jossang P. Hadi H. A. Sevent T. Bodo B. J. Org. Chem. 1991;56:6527. doi: 10.1021/jo00023a016. - DOI
- Shi J.-S. Yu J.-X. Chen X.-P. Xu R.-X. Acta Pharmacol. Sin. 2003;24:97–101. - PubMed
-
-
- Baeyer A. Knop C. A. Ann. Chem. Pharm. 1866;140:1. doi: 10.1002/jlac.18661400102. - DOI
-
-
For recent examples see:
- Zhang Y.-C. Jiang F. Shi F. Acc. Chem. Res. 2019;53:425. doi: 10.1021/acs.accounts.9b00549. - DOI - PubMed
- Basnet P. Sebold M. B. Hendrick C. E. Kozlowski M. C. Org. Lett. 2020;22:9524. doi: 10.1021/acs.orglett.0c03581. - DOI - PMC - PubMed
- Li G. Liu M. Zou S. Feng X. Lin L. Org. Lett. 2020;22:8708. doi: 10.1021/acs.orglett.0c03305. - DOI - PubMed
- Liang K. Li N. Zhang Y. Li T. Xia C. Chem. Sci. 2019;10:3049. doi: 10.1039/C8SC05170D. - DOI - PMC - PubMed
- Tetsuya S. Diachi H. Masaki T. Hidemi Y. Eur. J. Org. Chem. 2019;15:1813.
-
LinkOut - more resources
Full Text Sources

