Enantioselective synthesis of α-amino ketones through palladium-catalyzed asymmetric arylation of α-keto imines
- PMID: 35432891
- PMCID: PMC8966749
- DOI: 10.1039/d2sc00386d
Enantioselective synthesis of α-amino ketones through palladium-catalyzed asymmetric arylation of α-keto imines
Abstract
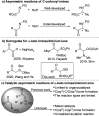
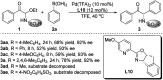
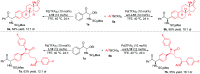
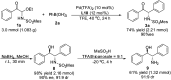
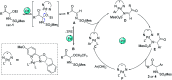
Chiral α-amino ketones are common structural motifs in natural products and pharmaceuticals, as well as important synthons in organic synthesis. Thus, establishing efficient methods for preparing compounds with these privileged scaffolds is an important endeavor in synthetic chemistry. Herein we disclose a new catalytic asymmetric approach for the synthesis of chiral α-amino ketones through a chiral palladium-catalyzed arylation reaction of in situ generated challenging α-keto imines from previously unreported C-acyl N-sulfonyl-N,O-aminals, with arylboronic acids. The current reaction offers a straightforward approach to the asymmetric synthesis of acyclic α-amino ketones in a practical and highly stereocontrolled manner. Meanwhile, the multiple roles of the chiral Pd(ii) complex catalyst in the reaction were also reported.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Chiral Pd-Catalyzed Enantioselective Syntheses of Various N-C Axially Chiral Compounds and Their Synthetic Applications.Acc Chem Res. 2021 Feb 2;54(3):719-730. doi: 10.1021/acs.accounts.0c00767. Epub 2021 Jan 22. Acc Chem Res. 2021. PMID: 33481580
-
Synthetic applications of nonmetal catalysts for homogeneous oxidations.Chem Rev. 2001 Nov;101(11):3499-548. doi: 10.1021/cr000019k. Chem Rev. 2001. PMID: 11840992
-
P-Chiral Phosphorus Ligands Based on a 2,3-Dihydrobenzo[ d][1,3]oxaphosphole Motif for Asymmetric Catalysis.Acc Chem Res. 2019 Apr 16;52(4):1101-1112. doi: 10.1021/acs.accounts.9b00029. Epub 2019 Mar 8. Acc Chem Res. 2019. PMID: 30848882
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
Cited by
-
Palladium-catalyzed asymmetric three-component reaction between glyoxylic acid, sulfonamides and arylboronic acids for the synthesis of α-arylglycine derivatives.Front Chem. 2023 Mar 13;11:1165618. doi: 10.3389/fchem.2023.1165618. eCollection 2023. Front Chem. 2023. PMID: 36993813 Free PMC article.
References
-
- Foley K. F. Cozzi N. V. Drug Dev. Res. 2003;60:252–260. doi: 10.1002/ddr.10297. - DOI
- Lin L.-Z. Cordell G. A. Ni C.-Z. Clardy J. Phytochemistry. 1991;30:1311–1315. doi: 10.1016/S0031-9422(00)95223-3. - DOI
- Kitajima M. Kogure N. Yamaguchi K. Takayama H. Aimi N. Org. Lett. 2003;5:2075–2078. doi: 10.1021/ol0344725. - DOI - PubMed
- Meltzer P. C. Butler D. Deschamps J. R. Madras B. K. J. Med. Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. - DOI - PMC - PubMed
- Myers M. C. Wang J. Iera J. A. Bang J. Hara T. Saito S. Zambetti G. P. Appella D. H. J. Am. Chem. Soc. 2005;127:6152–6153. doi: 10.1021/ja045752y. - DOI - PubMed
-
- de la Torre A. Tona V. Maulide N. Angew. Chem., Int. Ed. 2017;56:12416–12423. doi: 10.1002/anie.201702937. - DOI - PubMed
- Momiyama N. Yamamoto H. J. Am. Chem. Soc. 2004;126:5360–5361. doi: 10.1021/ja039103i. - DOI - PubMed
- Anada M. Tanaka M. Washio T. Yamawaki M. Abe T. Hashimoto S. Org. Lett. 2007;9:4559–4562. doi: 10.1021/ol702019b. - DOI - PubMed
- Takeda T. Terada M. J. Am. Chem. Soc. 2013;135:15306–15309. doi: 10.1021/ja408296h. - DOI - PubMed
- Yang X. Toste F. D. J. Am. Chem. Soc. 2015;137:3205–3208. doi: 10.1021/jacs.5b00229. - DOI - PMC - PubMed
- Li W. Liu X. Hao X. Hu X. Chu Y. Cao W. Qin S. Hu C. Lin L. Feng X. J. Am. Chem. Soc. 2011;133:15268–15271. doi: 10.1021/ja2056159. - DOI - PubMed
- Phukan P. Sudalai A. Tetrahedron: Asymmetry. 1998;9:1001–1005. doi: 10.1016/S0957-4166(98)00035-4. - DOI
- Liang J.-L. Yu X.-Q. Che C.-M. Chem. Commun. 2002:124–125. doi: 10.1039/B109272C. - DOI - PubMed
LinkOut - more resources
Full Text Sources

