Diazido macrocyclic sulfates as a platform for the synthesis of sequence-defined polymers for antibody drug conjugates
- PMID: 35432892
- PMCID: PMC8966716
- DOI: 10.1039/d1sc06242e
Diazido macrocyclic sulfates as a platform for the synthesis of sequence-defined polymers for antibody drug conjugates
Abstract
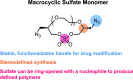
To improve the efficacy of antibody drug conjugates (ADCs), there has been significant focus on increasing the drug-to-antibody ratio (DAR) in order to deliver more payload. However, due to the hydrophobicity of many cytotoxics, highly-loaded conjugates often have lower physicochemical stability and poorer pharmacokinetic outcomes, requiring the development of new hydrophilic linkers. Herein, we report a platform for the preparation of functional, sequence-defined polymers for conjugation to antibodies. We demonstrate the successful synthesis of novel diazido macrocyclic sulfate monomers of varied size ranging from 4 to 7 ethylene glycol repeat units. These monomers were then successively ring-opened to produce sequence-defined polymers that contained either 4 or 6 azides for post-synthesis functionalization. Given the hydrophilic ethylene glycol backbone and chemically defined nature of the polymers, we envisioned this as a useful strategy in the preparation of highly-loaded ADCs. To demonstrate this, we prepared a model polymer-fluorophore scaffold composed of 4 coumarin molecules and conjugated it to Herceptin. We fully characterized the conjugate via mass spectrometry, which yielded a polymer-to-antibody ratio of 6.6, translating to a total of 26 fluorophores conjugated to the antibody at the inter-chain disulfides. We believe this technology to not only be a meaningful contribution to the field of sequence-defined polymers and conjugates, but also as a general and tunable platform for drug delivery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



Similar articles
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Polyethylene glycol-based linkers as hydrophilicity reservoir for antibody-drug conjugates.J Control Release. 2021 Sep 10;337:431-447. doi: 10.1016/j.jconrel.2021.07.041. Epub 2021 Jul 27. J Control Release. 2021. PMID: 34329685
-
Effects of Drug-Antibody Ratio on Pharmacokinetics, Biodistribution, Efficacy, and Tolerability of Antibody-Maytansinoid Conjugates.Bioconjug Chem. 2017 May 17;28(5):1371-1381. doi: 10.1021/acs.bioconjchem.7b00062. Epub 2017 Apr 13. Bioconjug Chem. 2017. PMID: 28388844
-
Practical approaches for overcoming challenges in heightened characterization of antibody-drug conjugates with new methodologies and ultrahigh-resolution mass spectrometry.MAbs. 2018 Apr;10(3):335-345. doi: 10.1080/19420862.2018.1433973. Epub 2018 Feb 20. MAbs. 2018. PMID: 29393747 Free PMC article. Review.
-
[Novel Chemical Linkers for Next-generation Antibody-drug Conjugates(ADCs)].Yakugaku Zasshi. 2019;139(2):209-219. doi: 10.1248/yakushi.18-00169-3. Yakugaku Zasshi. 2019. PMID: 30713230 Review. Japanese.
Cited by
-
Antibody Polymer Conjugates (APCs) for Active Targeted Therapeutic Delivery.Biomacromolecules. 2023 Aug 14;24(8):3638-3646. doi: 10.1021/acs.biomac.3c00385. Epub 2023 Jul 21. Biomacromolecules. 2023. PMID: 37478281 Free PMC article.
References
-
- Tumey L. N., in Innovations for Next-Generation Antibody-Drug Conjugates, ed. M. Damelin, Springer International Publishing, Cham, 2018, pp. 187–214
-
- Bodyak N. and Yurkovetskiy A. V., in Innovations for Next-Generation Antibody-Drug Conjugates, ed. M. Damelin, Springer International Publishing, Cham, 2018, pp. 215–240

