Acceptorless dehydrogenative synthesis of primary amides from alcohols and ammonia
- PMID: 35432908
- PMCID: PMC8966752
- DOI: 10.1039/d1sc07102e
Acceptorless dehydrogenative synthesis of primary amides from alcohols and ammonia
Abstract
The highly desirable synthesis of the widely-used primary amides directly from alcohols and ammonia via acceptorless dehydrogenative coupling represents a clean, atom-economical, sustainable process. Nevertheless, such a reaction has not been previously reported, and the existing catalytic systems instead generate other N-containing products, e.g., amines, imines and nitriles. Herein, we demonstrate an efficient and selective ruthenium-catalyzed synthesis of primary amides from alcohols and ammonia gas, accompanied by H2 liberation. Various aliphatic and aromatic primary amides were synthesized in high yields, with no observable N-containing byproducts. The selectivity of this system toward primary amide formation is rationalized through density functional theory (DFT) calculations, which show that dehydrogenation of the hemiaminal intermediate into primary amide is energetically favored over its dehydration into imine.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



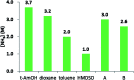

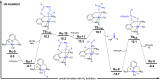
References
-
- Roundhill D. M. Chem. Rev. 1992;92:1–27. doi: 10.1021/cr00009a001. - DOI
- McCullough K. J., in Encyclopedia of Reagents for Organic Synthesis, ed. L. A. Paquette, Wiley: Chichester, U.K., 2001, ch. Ammonia
- Klinkenberg J. L. Hartwig J. F. Angew. Chem., Int. Ed. 2011;50:86–95. doi: 10.1002/anie.201002354. - DOI - PMC - PubMed
- Kim i. Kim H. J. Chang S. Eur. J. Org. Chem. 2013:3201–3213. doi: 10.1002/ejoc.201300164. - DOI
-
- Gunanathan C. Milstein D. Angew. Chem., Int. Ed. 2008;47:8661–8664. doi: 10.1002/anie.200803229. - DOI - PubMed
- Pingen D. Müller C. Vogt D. Angew. Chem., Int. Ed. 2010;49:8130–8133. doi: 10.1002/anie.201002583. - DOI - PubMed
- Ye X. Plessow P. N. Brinks M. K. Schelwies M. Schaub T. Rominger F. Paciello R. Limbach M. Hofmann P. J. Am. Chem. Soc. 2014;136:5923–5929. doi: 10.1021/ja409368a. - DOI - PubMed
- Balaraman E. Srimani D. Diskin-Posner Y. Milstein D. Catal. Lett. 2015;145:139–144. doi: 10.1007/s10562-014-1422-2. - DOI
- Fujita K.-i. Furukawa S. Morishima N. Shimizu M. Yamaguchi R. ChemCatChem. 2018;10:1993–1997. doi: 10.1002/cctc.201702037. - DOI
- Daw P. Ben-David Y. Milstein D. J. Am. Chem. Soc. 2018;140:11931–11934. doi: 10.1021/jacs.8b08385. - DOI - PMC - PubMed
- Daw P. Kumar A. Espinosa-Jalapa N. A. Ben-David Y. Milstein D. J. Am. Chem. Soc. 2019;141:12202–12206. doi: 10.1021/jacs.9b05261. - DOI - PubMed
- Wang Y. Furukawa S. Zhang Z. Torrente-Murciano L. Khan S. A. Yan N. Catal. Sci. Technol. 2019;9:86–96. doi: 10.1039/C8CY01799A. - DOI
- Wang Y. Furukawa S. Yan N. ACS Catal. 2019;9:6681–6691.
-
- Zhang J. Leitus G. Ben-David Y. Milstein D. J. Am. Chem. Soc. 2005;127:10840–10841. doi: 10.1021/ja052862b. - DOI - PubMed
- Gunanathan C. Ben-David Y. Milstein D. Science. 2007;317:790–792. doi: 10.1126/science.1145295. - DOI - PubMed
- Gnanaprakasam B. Zhang J. Milstein D. Angew. Chem., Int. Ed. 2010;49:1468–1471. doi: 10.1002/anie.200907018. - DOI - PubMed
- Balaraman E. Khaskin E. Leitus G. Milstein D. Nat. Chem. 2013;5:122–125. doi: 10.1038/nchem.1536. - DOI - PubMed
- Crabtree R. H. Chem. Rev. 2017;117:9228–9246. doi: 10.1021/acs.chemrev.6b00556. - DOI - PubMed
- Espinosa-Jalapa N. A. Kumar A. Leitus G. Diskin-Posner Y. Milstein D. J. Am. Chem. Soc. 2017;139:11722–11725. doi: 10.1021/jacs.7b08341. - DOI - PubMed
- Luo J. Rauch M. Avram L. Diskin-Posner Y. Shmul G. Ben-David Y. Milstein D. Nat. Catal. 2020;3:887–892. doi: 10.1038/s41929-020-00514-9. - DOI
-
- Zeng H. Guan Z. J. Am. Chem. Soc. 2011;133:1159–1161. doi: 10.1021/ja106958s. - DOI - PMC - PubMed
- Prechtl M. H. G. Wobser K. Theyssen N. Ben-David Y. Milstein D. Leitner W. Catal. Sci. Technol. 2012;2:2039–2042. doi: 10.1039/C2CY20429K. - DOI
- Srimani D. Balaraman E. Hu P. Ben-David Y. Milstein D. Adv. Synth. Catal. 2013;355:2525–2530. doi: 10.1002/adsc.201300620. - DOI
- Spasyuk D. Vicent C. Gusev D. G. J. Am. Chem. Soc. 2015;137:3743–3746. doi: 10.1021/ja512389y. - DOI - PubMed
- Zou Y.-Q. Zhou Q.-Q. Diskin-Posner Y. Ben-David Y. Milstein D. Chem. Sci. 2020;11:7188–7193. doi: 10.1039/D0SC02065F. - DOI - PMC - PubMed
- Kar S. Xie Y. Zhou Q.-Q. Diskin-Posner Y. Ben-David Y. Milstein D. ACS Catal. 2021;11:7383–7393. doi: 10.1021/acscatal.1c00728. - DOI - PMC - PubMed
-
- The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Material Science, ed. A. Greenberg, C. M. Breneman and J. F. Liebman, Wiley, New York, 2000
- Humphrey J. M. Chamberlin A. R. Chem. Rev. 1997;97:2243–2266. doi: 10.1021/cr950005s. - DOI - PubMed
- Bray B. L. Nat. Rev. Drug Discovery. 2003;2:587–593. doi: 10.1038/nrd1133. - DOI - PubMed
- Dineen T. A. Zajac M. A. Myers A. G. J. Am. Chem. Soc. 2006;128:16406–16409. doi: 10.1021/ja066728i. - DOI - PubMed
- Pattabiraman V. R. Bode J. W. Nature. 2011;480:471–479. doi: 10.1038/nature10702. - DOI - PubMed
- Szostak M. Spain M. Eberhart A. J. Procter D. J. J. Am. Chem. Soc. 2014;136:2268–2271. doi: 10.1021/ja412578t. - DOI - PMC - PubMed
- Ganesan M. Nagaraaj P. Org. Chem. Front. 2020;7:3792–3814. doi: 10.1039/D0QO00843E. - DOI
- Thakur R. Jaiswal Y. Kumar A. Tetrahedron. 2021;93:132313. doi: 10.1016/j.tet.2021.132313. - DOI

