8-Prenylnaringenin tissue distribution and pharmacokinetics in mice and its binding to human serum albumin and cellular uptake in human embryonic kidney cells
- PMID: 35432956
- PMCID: PMC9007292
- DOI: 10.1002/fsn3.2733
8-Prenylnaringenin tissue distribution and pharmacokinetics in mice and its binding to human serum albumin and cellular uptake in human embryonic kidney cells
Abstract
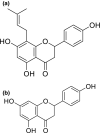
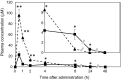
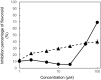
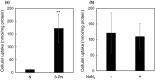
8-Prenylnaringenin (8-PN), a hop flavonoid, is a promising food substance with health benefits. Compared with nonprenylated naringenin, 8-PN exhibits stronger estrogenic activity and prevents muscle atrophy. Moreover, 8-PN prevents hot flushes and bone loss. Considering that prenylation reportedly improves the bioavailability of flavonoids, we compared the parameters related to the bioavailability [pharmacokinetics and tissue distribution in C57/BL6 mice, binding affinity to human serum albumin (HSA), and cellular uptake in HEK293 cells] of 8-PN and its mother (non-prenylated) compound naringenin. C57/BL6 mice were fed an 8-PN or naringenin mixed diet for 22 days. The amount of 8-PN (nmol/g tissue) in the kidneys (16.8 ± 9.20), liver (14.8 ± 2.58), muscles (3.33 ± 0.60), lungs (2.07 ± 0.68), pancreas (1.80 ± 0.38), heart (1.71 ± 0.27), spleen (1.36 ± 0.29), and brain (0.31 ± 0.09) was higher than that of naringenin. A pharmacokinetic study in mice demonstrated that the C max of 8-PN (50 mg/kg body weight) was lower than that of naringenin; however, the plasma concentration of 8-PN 8 h after ingestion was higher than that of naringenin. The binding affinity of 8-PN to HSA and cellular uptake in HEK293 cells were higher than those of naringenin. 8-PN bioavailability features assessed in mouse or human model experiments were obviously different from those of naringenin.
Keywords: 8‐prenylnaringenin; naringenin; pharmacokinetics; serum albumin; tissue accumulation.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
This study was partly funded by Dicel Corporation. Yuichi Ukawa and Kenichi Oe are employees of Dicel Corporation.
Figures
References
-
- Andres‐Lacueva, C. , Macarulla, M. T. , Rotches‐Ribalta, M. , Boto‐Ordóñez, M. , Urpi‐Sarda, M. , Rodríguez, V. M. , & Portillo, M. P. (2012). Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. Journal of Agriculture and Food Chemistry, 60(19), 4833–4840. 10.1021/jf3001108 - DOI - PubMed
-
- Askari, A. , Mokaberi, P. , Dareini, M. , Medalian, M. , Pejhan, M. , Erfani, M. , Asadzadeh‐Lotfabad, M. , Saberi, M.‐R. , & Chamani, J. (2021). Impact of linker histone in the formation of ambochlorin‐calf thymus DNA complex: Multi‐spectroscopic, stopped‐flow, and molecular modeling approaches. Iranian Journal of Basic Medical Sciences, 24(11), 1568–1582. 10.22038/ijbms.2021.58829.13070 - DOI - PMC - PubMed
-
- Bai, Y. , Peng, W. , Yang, C. , Zou, W. , Liu, M. , Wu, H. , Fan, L. , Li, P. , Zeng, X. , & Su, W. (2020). Pharmacokinetics and metabolism of Naringin and active metabolite Naringenin in rats, dogs, humans, and the differences between species. Frontiers in Pharmacology, 11, 364. 10.3389/fphar.2020.00364 - DOI - PMC - PubMed
-
- Barreca, D. , Laganà, G. , Toscano, G. , Calandra, P. , Kiselev, M. A. , Lombardo, D. , & Bellocco, E. ((2017). The interaction and binding of flavonoids to human serum albumin modify its conformation, stability and resistance against aggregation and oxidative injuries. Biochimica Et Biophysica Acta (BBA) ‐ General Subjects, 1861(1), 3531–3539. 10.1016/j.bbagen.2016.03.014 - DOI - PubMed
-
- Beigoli, S. , Sharifi Rad, A. , Askari, A. , Assaran Darban, R. , & Chamani, J. (2019). Isothermal titration calorimetry and stopped flow circular dichroism investigations of the interaction between lomefloxacin and human serum albumin in the presence of amino acids. Journal of Biomolecular Structure & Dynamics, 37(9), 2265–2282. 10.1080/07391102.2018.1491421 - DOI - PubMed
LinkOut - more resources
Full Text Sources

