Zebrafish obesogenic test identifies anti-adipogenic fraction in Moringa oreifera leaf extracts
- PMID: 35432980
- PMCID: PMC9007296
- DOI: 10.1002/fsn3.2758
Zebrafish obesogenic test identifies anti-adipogenic fraction in Moringa oreifera leaf extracts
Abstract
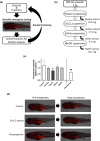
The zebrafish obesogenic test (ZOT) is a powerful tool for identifying anti-adipogenic compounds for in vivo screening. In our previous study, we found that Moringa oleifera (MO) leaf powder suppressed the accumulation of visceral adipose tissue (VAT) in ZOT. MO demonstrates a wide range of pharmacological effects; however, little is known about its functional constituents. To identify the anti-adipogenic components of MO leaves, we prepared extracts using different extraction methods and tested the obtained extracts and fractions using ZOT. We found that the dichloromethane extract and its hexane:EtOAc = 8:2 fraction reduced VAT accumulation in young zebrafish fed a high-fat diet. We also performed gene expression analysis in the zebrafish VAT and found that CCAAT/enhancer-binding protein beta and CCAAT/enhancer-binding protein delta (associated with early stages of adipogenesis) gene expression was downregulated after fraction 2 administration. We identified a new MO fraction that suppressed VAT accumulation by inhibiting early adipogenesis using the ZOT. Phenotype-driven zebrafish screening is a reasonable strategy for identifying bioactive components in natural products.
Keywords: diabetes; dyslipidemia; herbal medicine; natural products; visceral obesity.
© 2022 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
Youngil Kim, Djong‐Chi Chu, and Lekh Raj Juneja are employees of Rohto Pharmaceutical Co., Ltd.. The other authors declare no competing interests.
Figures


References
-
- Ahmad, J. , Khan, I. , Johnson, S. K. , Alam, I. , & Din, Z. U. (2018). Effect of incorporating stevia and Moringa in cookies on postprandial glycemia, appetite, palatability, and gastrointestinal well‐being. Journal of the American College of Nutrition, 37(2), 133–139. 10.1080/07315724.2017.1372821 - DOI - PubMed
-
- Almatrafi, M. M. , Vergara‐Jimenez, M. , Murillo, A. G. , Norris, G. H. , Blesso, C. N. , & Fernandez, M. L. (2017). Moringa leaves prevent hepatic lipid accumulation and inflammation in guinea pigs by reducing the expression of genes involved in lipid metabolism. International Journal of Molecular Sciences, 18(7), 1330. 10.3390/ijms18071330 - DOI - PMC - PubMed
-
- Balakrishnan, B. B. , Krishnasamy, K. , & Choi, K. C. (2018). Moringa concanensis Nimmo ameliorates hyperglycemia in 3T3‐L1 adipocytes by upregulating PPAR‐γ, C/EBP‐α via Akt signaling pathway and STZ‐induced diabetic rats. Biomedicine and Pharmacotherapy, 103, 719–728. 10.1016/j.biopha.2018.04.047 - DOI - PubMed
-
- Busquet, F. , Strecker, R. , Rawlings, J. M. , Belanger, S. E. , Braunbeck, T. , Carr, G. J. , Cenijn, P. , Fochtman, P. , Gourmelon, A. , Hübler, N. , Kleensang, A. , Knöbel, M. , Kussatz, C. , Legler, J. , Lillicrap, A. , Martínez‐Jerónimo, F. , Polleichtner, C. , Rzodeczko, H. , Salinas, E. , … Halder, M. (2014). OECD validation study to assess intra‐ and inter‐laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regulatory Toxicology and Pharmacology, 69(3), 496–511. 10.1016/j.yrtph.2014.05.018 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

