Au(I) Catalyzed HF Transfer: Tandem Alkyne Hydrofluorination and Perfluoroarene Functionalization
- PMID: 35433106
- PMCID: PMC9007466
- DOI: 10.1021/acscatal.1c05474
Au(I) Catalyzed HF Transfer: Tandem Alkyne Hydrofluorination and Perfluoroarene Functionalization
Abstract
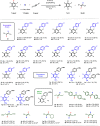
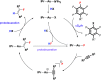
HF transfer reactions between organic substrates are potentially useful transformations. Such reactions require the development of catalytic systems that can promote both defluorination and fluorination steps in a single reaction sequence. Herein, we report a catalytic protocol in which an equivalent of HF is generated from a perfluoroarene | nucleophile pair and transferred directly to an alkyne. The reaction is catalyzed by [Au(IPr)NiPr2] (IPr = N,N'-1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene). HF transfer generates two useful products in the form of functionalized fluoroarenes and fluoroalkenes. Mechanistic studies (rate laws, KIEs, density functional theory (DFT) calculations, competition experiments) are consistent with the Au(I) catalyst facilitating a catalytic network involving both concerted SNAr and hydrofluorination steps. The nature of the nucleophile impacts the turnover-limiting step. The cSNAr step is turnover-limiting for phenol-based nucleophiles, while protodeuaration likely becomes turnover-limiting for aniline-based nucleophiles. The approach removes the need for direct handling of HF reagents in hydrofluorination and offers possibilities to manipulate the fluorine content of organic molecules through catalysis.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
