Iron Administration Overcomes Resistance to Erastin-Mediated Ferroptosis in Ovarian Cancer Cells
- PMID: 35433479
- PMCID: PMC9008715
- DOI: 10.3389/fonc.2022.868351
Iron Administration Overcomes Resistance to Erastin-Mediated Ferroptosis in Ovarian Cancer Cells
Abstract
Objectives: Developing novel therapeutic approaches to defeat chemoresistance is the major goal of ovarian cancer research. Induction of ferroptosis has shown promising antitumor effects in ovarian cancer cells, but the existence of still undefined genetic and metabolic determinants of susceptibility has so far limited the application of ferroptosis inducers in vivo.
Methods: Erastin and/or the iron compound ferlixit were used to trigger ferroptosis in HEY, COV318, PEO4, and A2780CP ovarian cancer cell lines. Cell viability and cell death were measured by MTT and PI flow cytometry assay, respectively. The "ballooning" phenotype was tested as ferroptosis specific morphological feature. Mitochondrial dysfunction was evaluated based on ultrastructural changes, mitochondrial ROS, and mitochondrial membrane polarization. Lipid peroxidation was tested through both C11-BODIPY and malondialdehyde assays. VDAC2 and GPX4 protein levels were quantified as additional putative indicators of mitochondrial dysfunction or lipid peroxidation, respectively. The effect of erastin/ferlixit treatments on iron metabolism was analyzed by measuring intracellular labile iron pool and ROS. FtH and NCOA4 were measured as biomarkers of ferritinophagy.
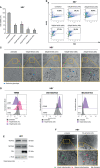
Results: Here, we provide evidence that erastin is unable to induce ferroptosis in a series of ovarian cancer cell lines. In HEY cells, provided with a high intracellular labile iron pool, erastin treatment is accompanied by NCOA4-mediated ferritinophagy and mitochondrial dysfunction, thus triggering ferroptosis. In agreement, iron chelation counteracts erastin-induced ferroptosis in these cells. COV318 cells, with low baseline intracellular labile iron pool, appear resistant to erastin treatment. Notably, the use of ferlixit sensitizes COV318 cells to erastin through a NCOA4-independent intracellular iron accumulation and mitochondrial dysfunction. Ferlixit alone mimics erastin effects and promotes ferroptosis in HEY cells.
Conclusion: This study proposes both the baseline and the induced intracellular free iron level as a significant determinant of ferroptosis sensitivity and discusses the potential use of ferlixit in combination with erastin to overcome ferroptosis chemoresistance in ovarian cancer.
Keywords: chemoresistance; erastin; ferroptosis; iron; mitochondrial dysfunction; ovarian cancer.
Copyright © 2022 Battaglia, Sacco, Perrotta, Faniello, Scalise, Torella, Levi, Costanzo and Biamonte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

