Autophagy Modulators in Coronavirus Diseases: A Double Strike in Viral Burden and Inflammation
- PMID: 35433503
- PMCID: PMC9010404
- DOI: 10.3389/fcimb.2022.845368
Autophagy Modulators in Coronavirus Diseases: A Double Strike in Viral Burden and Inflammation
Abstract
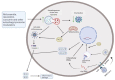
Coronaviruses are the etiologic agents of several diseases. Coronaviruses of critical medical importance are characterized by highly inflammatory pathophysiology, involving severe pulmonary impairment and infection of multiple cell types within the body. Here, we discuss the interplay between coronaviruses and autophagy regarding virus life cycle, cell resistance, and inflammation, highlighting distinct mechanisms by which autophagy restrains inflammatory responses, especially those involved in coronavirus pathogenesis. We also address different autophagy modulators available and the rationale for drug repurposing as an attractive adjunctive therapy. We focused on pharmaceuticals being tested in clinical trials with distinct mechanisms but with autophagy as a common target. These autophagy modulators act in cell resistance to virus infection and immunomodulation, providing a double-strike to prevent or treat severe disease development and death from coronaviruses diseases.
Keywords: autophagy; coronaviral infection; inflammation; tissue damage; viral replication.
Copyright © 2022 Silva, Ribeiro, da Silva, da Costa and Travassos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alosaimi B., Hamed M. E., Naeem A., Alsharef A. A., AlQahtani S. Y., AlDosari K. M., et al. (2020). MERS-CoV Infection Is Associated With Downregulation of Genes Encoding Th1 and Th2 Cytokines/Chemokines and Elevated Inflammatory Innate Immune Response in the Lower Respiratory Tract. Cytokine 126, 154895. doi: 10.1016/j.cyto.2019.154895 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

