Immunomodulation as a Protective Strategy in Chronic Otitis Media
- PMID: 35433505
- PMCID: PMC9005906
- DOI: 10.3389/fcimb.2022.826192
Immunomodulation as a Protective Strategy in Chronic Otitis Media
Abstract
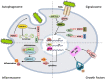
Introduction: Major features of the pathogenesis in otitis media, the most common disease in childhood, include hyperplasia of the middle ear mucosa and infiltration by leukocytes, both of which typically resolve upon bacterial clearance via apoptosis. Activation of innate immune receptors during the inflammatory process leads to the activation of intracellular transcription factors (such as NF-κB, AP-1), which regulate both the inflammatory response and tissue growth. We investigated these leading signaling pathways in otitis media using mouse models, human samples, and human middle ear epithelial cell (HMEEC) lines for therapeutic immunomodulation.
Methods: A stable otitis media model in wild-type mice and immunodeficient KO-mice, as well as human tissue samples from chronic otitis media, skin from the external auditory canal and middle ear mucosa removed from patients undergoing ear surgery, were studied. Gene and protein expression of innate immune signaling molecules were evaluated using microarray, qPCR and IHC. In situ apoptosis detection determined the apoptotic rate. The influence of bacterial infection on immunomodulating molecules (TNFα, MDP, Tri-DAP, SB203580, Cycloheximide) in HMEEC was evaluated. HMEEC cells were examined after bacterial stimulation/inhibition for gene expression and cellular growth.
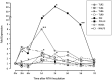
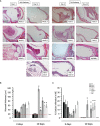
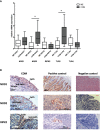
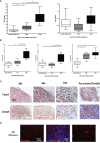
Results: Persistent mucosal hyperplasia of the middle ear mucosa in chronic otitis media resulted from gene and protein expression of inflammatory and apoptotic genes, including NODs, TNFα, Casp3 and cleaved Casp3. In clinical chronic middle ear samples, these molecules were modulated after a specific stimulation. They also induced a hyposensitive response after bacterial/NOD-/TLR-pathway double stimulation of HMEEC cells in vitro. Hence, they might be suitable targets for immunological therapeutic approaches.
Conclusion: Uncontrolled middle ear mucosal hyperplasia is triggered by TLRs/NLRs immunoreceptor activation of downstream inflammatory and apoptotic molecules.
Keywords: NOD-like receptors; TNFα; TOLL-like receptors; apoptosis; immunology; otitis media.
Copyright © 2022 Leichtle, Kurabi, Leffers, Därr, Draf, Ryan and Bruchhage.
Conflict of interest statement
AR is a co-founder of Otonomy Inc., serves as a member of the Scientific Advisory Board, and holds an equity position in the company. The UCSD Committee on Conflict of Interest has approved this relationship. Otonomy, Inc. played no part in the research reported here. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chonmaitree T., Alvarez-Fernandez P., Jennings K., Trujillo R., Marom T., Loeffelholz M. J., et al. . (2015). Symptomatic and Asymptomatic Respiratory Viral Infections in the First Year of Life: Association With Acute Otitis Media Development. Clin. Infect. Dis. 60 (1), 1–9. doi: 10.1093/cid/ciu714 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

